Abstract:
Recently, Prof. Shou-Fei Zhu’s research group at Nankai University reported a highly efficient and selective hydro-silylation reaction of asymmetric internal alkynes with trisubstituted silanes, catalyzed by a cobalt complex bearing a cyclopropane-based diphosphine ligand. The reaction proceeds under mild conditions, demonstrates broad substrate scope and excellent functional group tolerance, and is generally applicable for the synthesis of alkenylsilanes. Notably, the reaction displays a unique additive effect, where the regioselectivity reverses depending on the additive used. Mechanistic studies revealed that different additives alter the spin state of the catalyst during the reaction, leading to divergent regioselectivities. This study provides new insights into the rational application of additives in catalysis.
Background:
Controlling selectivity in catalytic reactions is a central challenge in organic synthesis. Reaction selectivity—including chemoselectivity, regioselectivity, and stereoselectivity—is typically governed by the steric and electronic properties of the catalyst and substrate. However, numerous studies have shown that additives can significantly influence reaction selectivity. In some cases, different additives lead to different selective outcomes, suggesting that additives represent a potentially powerful tool for tuning reaction pathways. Understanding the mechanisms behind these additive effects is therefore of great value in reaction design. Despite their importance, systematic studies on additive effects remain scarce, and their mechanistic roles are still poorly understood.
In recent years, Prof. Zhu’s team at Nankai University has focused on the development of organic reactions promoted by open-shell catalysts. They have developed a series of ligands based on phenanthroline and cyclopropane scaffolds and their corresponding iron and cobalt complexes. These catalysts have been applied in various hydrofunctionalization, carbon–zinc bond formation, and carbon–carbon coupling reactions. Their research highlighted the irreplaceable role of open-shell iron and cobalt catalysts, which is closely related to their spin states, leading to the proposal of the concept of “spin-responsive catalysis” to explore spin as a new dimension in catalysis.
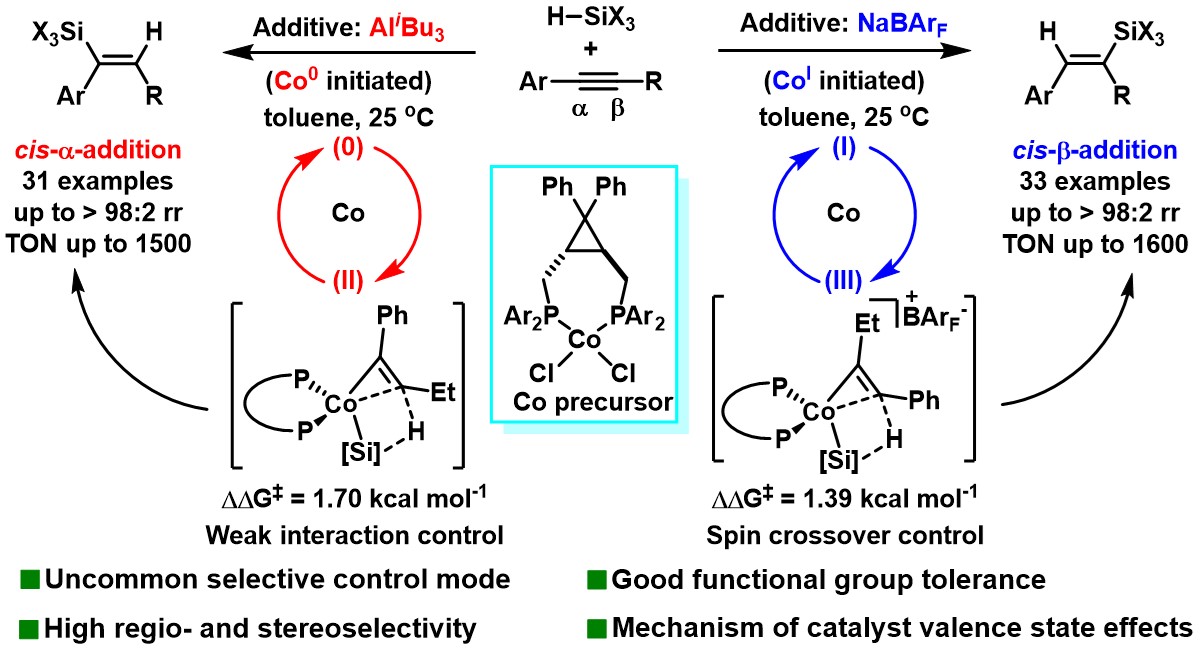
Figure 1. Additive-controlled regiodivergent hydrosilylation of alkynes
Highlights:
In this study, the authors report a cobalt-catalyzed hydrosilylation of asymmetric internal alkynes with trisubstituted silanes using a cyclopropane-based diphosphine ligand. The reaction proceeds efficiently under mild conditions, shows excellent functional group tolerance, high activity, and broad substrate applicability, providing a valuable method for synthesizing alkenylsilanes.
The reaction exhibits a striking additive effect: regioselectivity is completely reversed depending on the activator. In the hydrosilylation of aryl-alkyl internal alkynes, the use of AliBu₃ as an activator results in cis-α addition selectivity, whereas NaBArF leads to cis-β addition selectivity. Mechanistic studies indicate that different additives generate catalytically active cobalt species in distinct oxidation states, which correspond to different spin multiplicities and ultimately result in divergent regioselectivities.
Moreover, the combination of the sterically hindered gem-diaryl cyclopropane diphosphine ligand with the small-radius 3d metal cobalt forms a compact reaction cavity, allowing for subtle discrimination between substituents at both ends of the alkyne, thus ensuring excellent regioselectivity.
Conclusion and Outlook:
In summary, this work reports a cobalt-catalyzed hydrosilylation of alkynes that proceeds under mild conditions with high efficiency, broad substrate scope, and excellent functional group compatibility, showing strong application potential. The reaction’s regioselectivity is predominantly governed by the additive: AliBu₃ promotes cis-α selectivity, while NaBArF induces cis-β selectivity. Mechanistic investigations show that the differing oxidation states of cobalt catalysts induced by different additives lead to distinct spin states during the catalytic cycle, ultimately determining the regioselective outcome.
This study not only provides an efficient method for the synthesis of trisubstituted alkenylsilanes but also reveals a novel additive effect, introducing a new strategy where additives control regioselectivity by modulating the metal catalyst’s oxidation and spin states. This offers fresh perspectives for catalyst and reaction design.
This research was recently published online in CCS Chemistry as a Research Article. Dr. Yan-Dong Zhang and Ph.D. candidate Xiang-Yu Liu of Nankai University are co-first authors, and Prof. Shou-Fei Zhu is the corresponding author.
Article Details:
Additive-Controlled Regiodivergent Catalytic Alkyne Hydrosilylation Reactions
Yan-Dong Zhang†, Xiang-Yu Liu†, Peng He, Qiao Zhang, Ming-Yao Huang, and Shou-Fei Zhu*
Cite this by DOI: 10.31635/ccschem.025.202405338
Article Link: https://doi.org/10.31635/ccschem.025.202405338