Sulfur, from daily life to national strategy, is omnipresent. Yet sulfur chemistry faces numerous challenges and urgently requires a new green, efficient, and sustainable system to transition fully from the laboratory to industrial application.
The Unique Charm of Sulfur
Sulfur, element 16 on the periodic table, possesses distinctive properties due to its electronic configuration and orbital effects:
- An outer shell with six electrons, allowing oxidation states ranging from –2 to +6;
- Ability to form various bonds, such as single bonds (S–S) and double bonds (C=S);
- A relatively large atomic radius, facilitating the formation of long chains and polysulfide compounds.
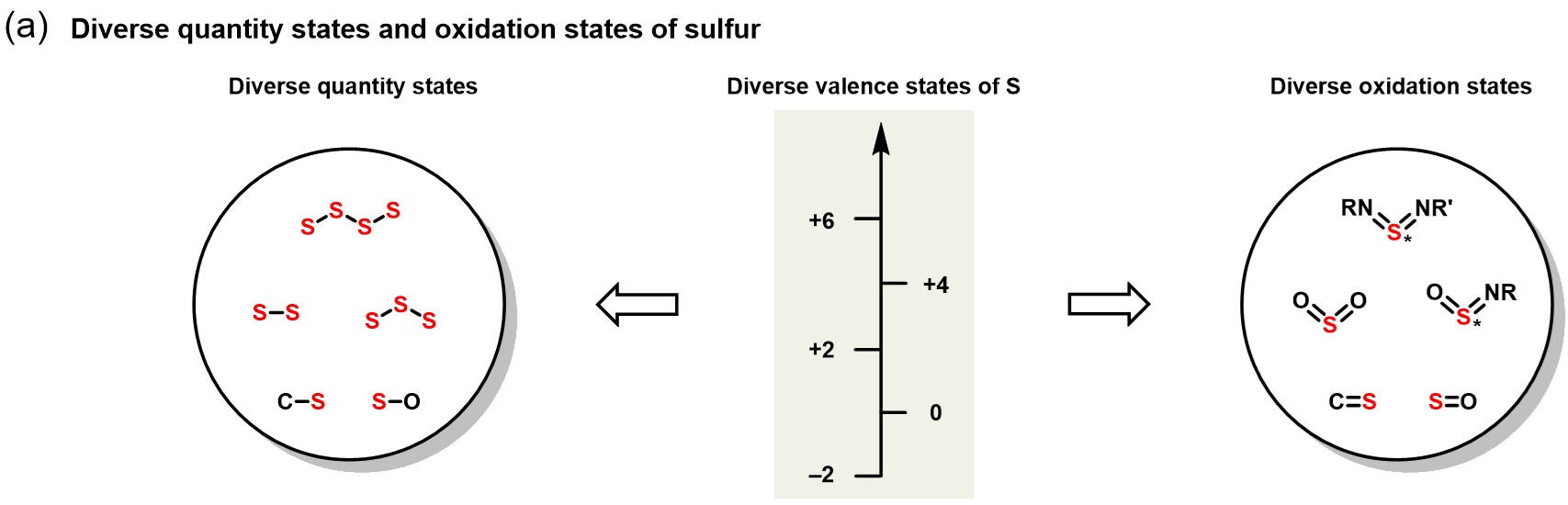
Sulfur's Quantity States and Oxidation States
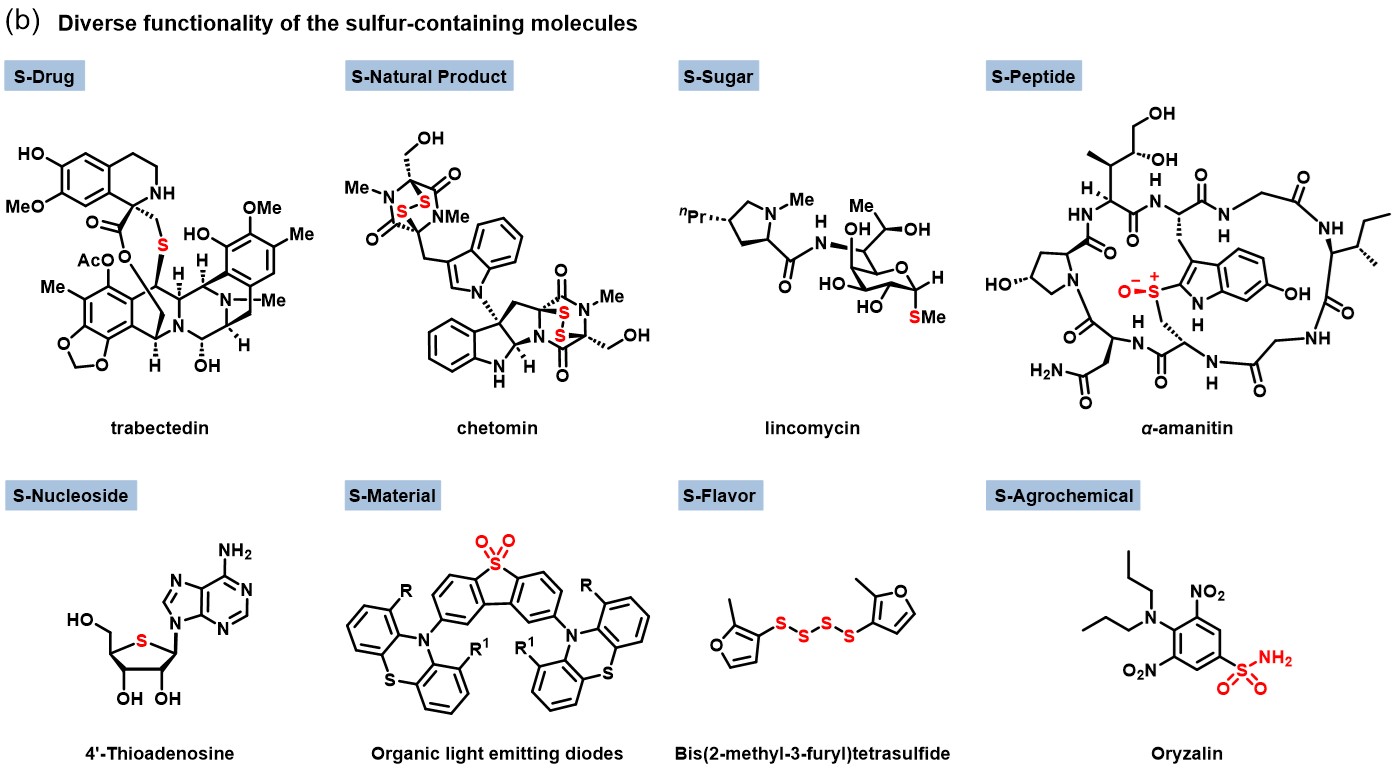
Sulfur's unique electronic structure gives rise to diverse quantity states and multidimensional oxidation states. This underpins its wide applications in sulfur-containing drugs, peptides, sugars, nucleosides, natural products, pesticides, fragrances, and materials. However, precise control and efficient incorporation of these quantity and oxidation states remain significant challenges.
Four Major Challenges in Sulfur Chemistry
Despite its vast potential, sulfur chemistry confronts several formidable
- Odor Issues: Most sulfur compounds (thiols, thiophenols, thioethers) have strong, unpleasant odors, making experimental handling difficult;
- Catalyst Poisoning: Sulfur’s lone electron pairs readily coordinate with metal catalysts, halting catalytic cycles;
- Difficulty in Polysulfide Construction: Reversible bond dynamics hinder precise control of sulfur chain length;
- Challenges in Introducing High-Valent Sulfur: Numerous side reactions occur due to multiple oxidation states, complicating control of sulfur valence.
The "Stumbling Blocks" to Industrialization
Industrialization of sulfur chemistry faces three main hurdles:
- Harsh Production Environments: Offensive odors lead to environmental and health risks;
- Difficult Process Control: High reactivity leads to many byproducts, making purification challenging;
- High Storage and Transport Costs: Unstable sulfur compounds require specialized packaging and cold-chain logistics. These factors keep many promising sulfur compounds confined to lab research.

From Inorganic to Organic Sulfur Introduction
To address scientific and industrial challenges in "greening sulfur chemistry," Professor Xuefeng Jiang’s group at East China Normal University proposed the innovative "mask effect" and established a scientific system for transforming inorganic sulfur into organic sulfur, achieving systematic breakthroughs in:
- Controllable quantity states
- Economical oxidation states
- The "Mask Effect"
- Electronic Regulation: Functional ionic groups in inorganic salts reduce sulfur electron density, solving catalyst poisoning;
- Steric Hindrance: Bulky groups shield vacant orbitals, preventing sulfur self-coupling side reactions;
- Orbital Delocalization: Conjugated systems disperse electron clouds, enhancing intermediate stability;
- Adjustable Solubility, Concentration, and Dipole Effects;
- Essentially, "putting a mask on sulfur" makes sulfur compounds odorless, safe, and easy to transport.
- "From Inorganic to Organic Sulfur Conversion"
By leveraging the "mask effect," traditionally odorous and catalyst-poisoning thiol-based sulfur sources are converted into odorless, stable, green, safe, and affordable inorganic sulfur salts, achieving:
- Operational Convenience: Odorless, stable, solid sulfur reagents that are easy to obtain, transport, and store;
- In Situ Release: Controlled release of reactive sulfur species during reactions;
- Directed Transfer: Precise incorporation of sulfur atoms via molecular recognition;
- This preserves reactivity while overcoming instability.
- “Controllable Quantity States”
Using "masked polysulfides", the team designed stable and tunable multi-sulfur reagents via electronic effects, steric hindrance, and dipole tension. This offers an economical and precise solution to challenges in constructing polysulfides (e.g., S–S, S–S–S, S–S–S–S).
- “Economical Oxidation States”
By introducing masked high-valent sulfur, industrial waste gases like SO₂ are substituted with industrial sulfur salts (e.g., sodium metabisulfite, sodium dithionite, thiourea dioxide, Rongalite) to enable controlled release and incorporation:
Avoid Redox Issues: Maintain oxidation states for redox economy; Parallel Oxygenation Pathways: Use of abundant SO₂ salts + metal catalysis + radical/ionic combinations; Diverse Stereocontrol: Construct chiral sulfur centers in oxo (O=S=O) and imino (O=S=NR, RN=S=NR') forms; Life-Linking Applications: "Click" chemistry using high-valent sulfur for bioconjugation.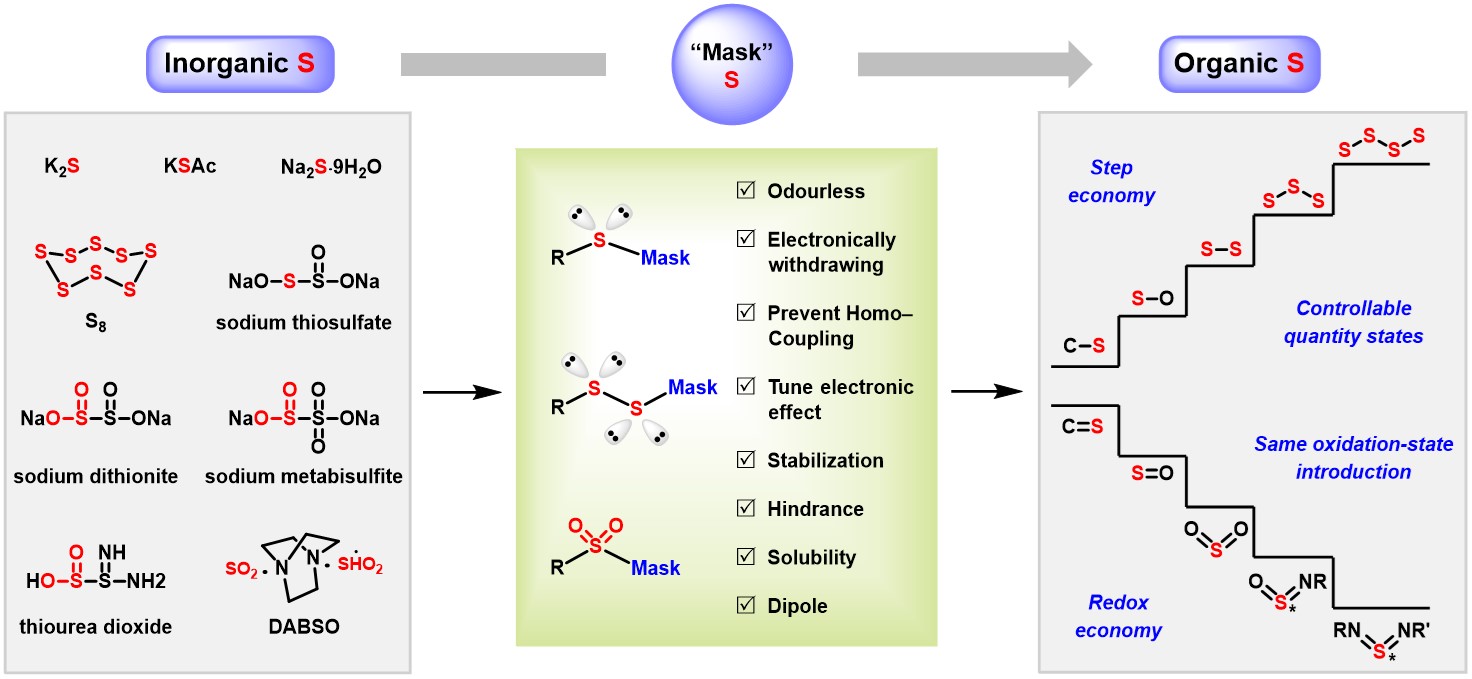
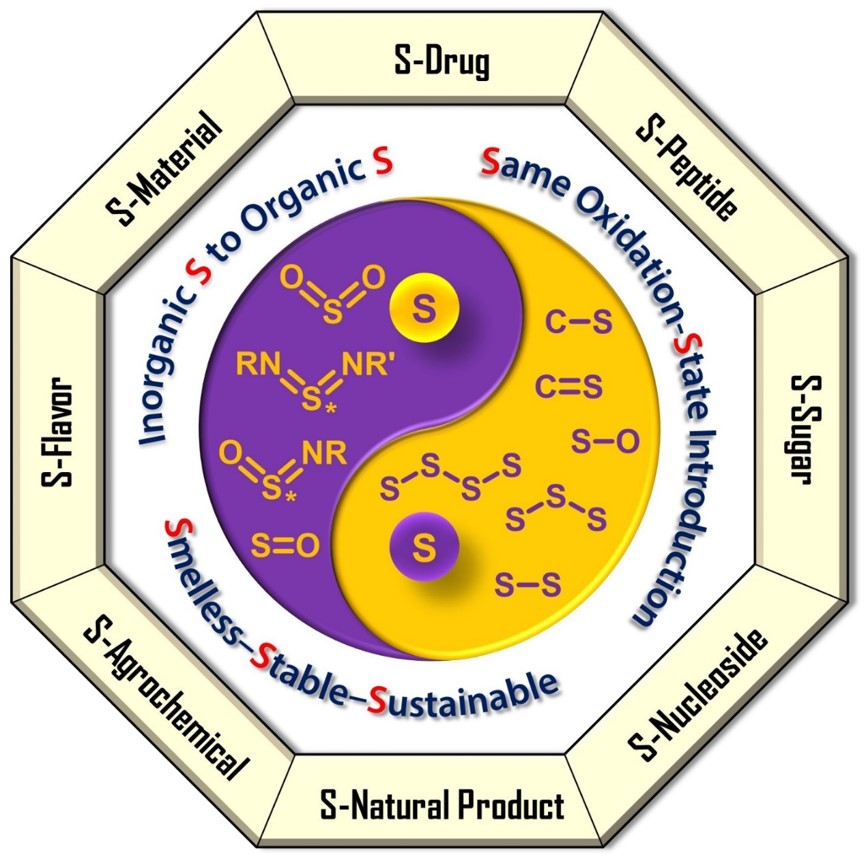
"3S" Green Sulfur Chemistry
Based on this, Jiang’s team established the "3S" (Smelless / Stable / Sustainable) green sulfur chemistry system:
- Smelless: Affordable, odorless sulfur salts;
- Stable: Safe, easy to store and transport;
- Sustainable: High economic efficiency and environmental friendliness.
This system has been widely recognized internationally and has become a new paradigm in academic and industrial sulfur chemistry. It addresses the construction of numerous sulfur-containing bond types across quantity and oxidation states, such as C–S, C=S, S–X, S–S, S–S–S, S–S–S–S, S–O, S=O, O=S=O, O=S=NR, and RN=S=NR'.
It has enabled the development of:
- Sulfur drugs (e.g., trabectedin),
- Sulfur peptides (e.g., polysulfur-linked peptides),
- Sulfur sugars (with α/β control),
- Sulfur nucleosides (e.g., thiomethyl modifications),
- Sulfur natural products (e.g., controllable domino dimerizations),
- Sulfur pesticides (green sulfonamide processes),
- Sulfur fragrances and flavors (precise polysulfide constructions),
- Sulfur materials (e.g., for optoelectronics and semiconductors).
The Future of Sulfur Chemistry
From foul-smelling and hard to control to green and precisely engineered, sulfur chemistry is undergoing a historic transformation. The "3S" green sulfur chemistry system not only overcomes fundamental scientific bottlenecks but also inspires innovations in pharmaceuticals, materials, and energy storage. As academia and industry integrate more deeply, "3S" sulfur chemistry is poised to catalyze the creation of original drugs, functional materials, and storage systems—benefiting human health and quality of life.
This work was published as a Mini Review in CCS Chemistry, with Professor Xuefeng Jiang of East China Normal University as the corresponding author.
Article Details:
Smelless/Stable/Sustainable Sulfur Chemistry
Leiyang Bai, Xuefeng Jiang*
Cite This by DOI: 10.31635/ccschem.025.202505568
Article Link: https://doi.org/10.31635/ccschem.025.202505568