Chemical Strategies of Tailoring PEDOT:PSS for Bioelectronic Applications: Synthesis, Processing and Device Fabrication
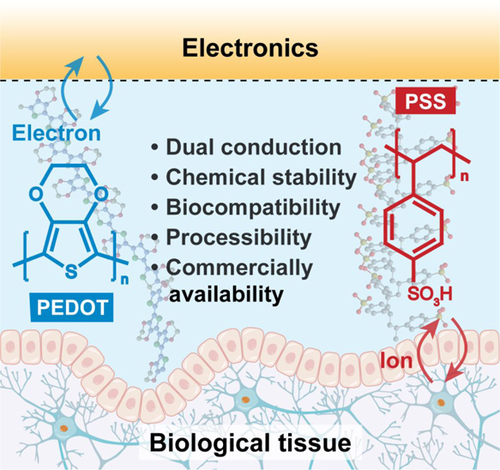
Poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) has been the most prominent conducting polymer due to its outstanding electrical properties, chemical stability, biocompatibility, and commercial availability. In this mini review, we aim to comprehensively outline the chemical approaches employed in tailoring PEDOT:PSS for bioelectronic applications. We open our discussion by showcasing various synthetic techniques and commercially accessible forms of PEDOT:PSS, providing practical advice and approaches to greatly enhance its electrical properties, and presenting diverse chemical designs and processing methods that are essential for converting PEDOT:PSS into different form factors, such as fibers, gels, and films, for integration a range of device structures. Additionally, we explore several burgeoning applications of PEDOT:PSS in bioelectronics, ranging from wearable health monitoring to implantable neural interfaces, underscoring its essential impact on improving device efficiency and biological compatibility, as it opens avenues for innovative diagnostic and therapeutic techniques in the realm of precision medicine. Concluding with an outlook, the review presents insights into the ongoing challenges and future research paths for PEDOT:PSS in the ever-evolving landscape of bioelectronics. We emphasize the need for continued innovation in materials science and engineering to further harness the full potential of this dynamic domain.
Introduction
Bioelectronics is transforming healthcare through innovative material and device designs, enabling accurate physiological monitoring and effective medical treatment. Traditional electronic devices typically rely on rigid inorganic materials such as metals, silicon, or carbon for human–machine interface. While techniques through structural designs and advanced fabrication can render rigid electronic devices in more flexible form factors, the inherit mismatch of material moduli (GPa for inorganic materials vs kPa for biological tissues) not only restricts the natural motions of these organs but can also lead to discomfort, irritation, and adverse immune responses at the interface, posing significant challenges to biocompatibility and long-term functionality.1 Besides the mechanical constraints, in terms of electrical properties, existing metal electrodes have a fundamental challenge in achieving low interfacial impedance and high charge injection capacity, which are critical factors for high-fidelity recording and effective delivery of electrical stimulation. Briefly, since metal electrodes are exclusively electronically conductive whereas biological tissues are solely ionically conductive, there always exists a Helmholtz double layer capacitor at the electrode-tissue interface, which dominates the interfacial impedance. The high impedance is a particularly severe issue when smaller electrodes are needed in high-resolution arrays for cellular level studies.2,3 To overcome the abovementioned limitations of rigid materials for traditional electronic devices, a conducting polymer, poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS), emerges as a promising alternative that can be seamlessly integrated with soft biological tissues with several unique advantages. First, in comparison to rigid inorganic materials, PEDOT:PSS has a much lower Young’s modulus (∼1–2 GPa) that can be further reduced through molecular designs to minimize tissue damage or foreign body response. Matching tissue-level modulus, PEDOT:PSS enables effective signal exchange through conformal tissue-electronics interfacing. Second, PEDOT:PSS is a mixed ionic/electronic conductor, providing a unique volumetric capacitance at the electrode-tissue interface, resulting in a considerable reduction in overall impedance compared to metal electrodes.4,5 In the meantime, the charge injection capacity of PEDOT:PSS is also significantly higher than that of metal, allowing for effective stimulation. Further, PEDOT:PSS has excellent biocompatibility and environmental stability. Under repetitive electrical stimulation in biological environments, PEDOT:PSS is able to resist performance degradation in the oxidative environment whereas noble metal like gold cannot survive in the long term. Compared to other conducting polymers, for example, polyaniline6 or polypyrrole,7 PEDOT:PSS distinguishes itself due to its excellent tunability through chemical strategies, outstanding electrical properties, excellent stability, and commercial availability. Taken together, PEDOT:PSS has gained increasing attention in bioelectronic applications as an ideal biointerfacing electronic material.
The versatility of PEDOT:PSS allows a vast array of chemical modifications and processing techniques. Extensive research has demonstrated that the conductivity of PEDOT:PSS can be substantially improved by various treatment methods. These include physical treatments (e.g., shearing induced alignment),8 blending with secondary dopants such as polar molecules, ionic liquids, surfactants, salts, zwitterions,9 and chemical post-treatments using acids or organic solvents.10,11 While conducting polymers often exhibit a trade-off between their mechanical and electrical properties, innovative design strategies have been developed to synergistically boost both the stretchability and conductivity of PEDOT:PSS. The adaptability of PEDOT:PSS also allows customization into multidimensional form factors such as fibers, gels, and films, which are adaptable to diverse device structures. This versatility is further augmented by the development of specialized fabrication strategies and processing techniques, leading to the tailored adaptations of PEDOT:PSS for optimal integration into bioelectronic devices.
Fibers, known for their lightness, flexibility, and skin compatibility, can be woven into smart textiles and stretchable sensors for monitoring physiological signals like heart rate or respiration. However, poor mechanical robustness remains a challenge for PEDOT fibers. Gels have tissue-level softness (0.5–500 kPa), good stretchability, and even adhesion to track subtle physical movements or electrophysiological signals. Yet, their poor electrical properties and limited processability hurdle the formation of high-density and small-scale features. Films are more prevalent in devices due to the high conductivity and the ease of fabricating high-density structures. In spite of this, thin film devices can experience cracks when exposed to strain or scratches from regular wear and tear. Due to the varying advantages and disadvantages of different forms of PEDOT, a thorough examination of the form factor and a comprehensive consideration of its chemical structure, miscibility, and compatibility with other components enables novel approaches to tackling obstacles in bioelectronic devices.
Herein, we highlight the critical role of chemical methods in realizing the full potential of PEDOT:PSS-based medical devices. Specifically, we emphasize material design strategies to endow pristine PEDOT:PSS with new properties like toughness, stretchability, low modulus, and tissue adhesion. These enhancements allow for the adaptation of PEDOT:PSS into diverse forms for further integration into bioelectronic devices, broadening its application scopes. Additionally, we also summarize and categorize the primary applications of PEDOT:PSS (e.g., chemical sensors, physical sensors, electrophysiological sensors, electrical stimulators), outlining its critical role in enhancing the performance of individual devices. This encompasses the areas where PEDOT:PSS are most effectively utilized, demonstrating its significant impact on the functionality and efficiency of bioelectronic devices. Finally, the review concludes by examining the current challenges and potential advancements in the rapidly evolving field of bioelectronics.
Basics of PEDOT:PSS
Chemical synthesis of PEDOT:PSS and its derivatives
Of all the forms of PEDOT, the most well-known is PEDOT complexed with PSS (Figure 1a), where PSS serves as a counter ion stabilizing the doped PEDOT and provides a matrix to disperse PEDOT in deprotonation. The polymerization mechanism can be categorized into three types: chemical polymerization, electrochemical polymerization, and transition metal-mediated coupling polymerization. The oxidative polymerization involves three steps (Figure 1b). First, the 3,4-ethylenedioxythiophene (EDOT) is oxidized to the corresponding free radical cation. Then, further oxidation and recombination of the dimers lead to the formation of PEDOT oligomers or polymers. Finally, the neutral PEDOT is doped by the oxidants, with the anions of the oxidants serving as counterions to stabilize the charged PEDOT. Oxidants influence the conductivity and water dispersibility of the resulting product. For example, strategies to reduce the activity of oxidant enhance conductivity of the polymerization product by moderating the reaction rate.12,13 Iron (III)-chloride oxidizes EDOT into insoluble powders where iron (III)-sulfonates result in products with good water dispersibility.14,15 Electrochemical polymerization is similar to oxidative polymerization except that EDOT is oxidized by an applied potential. For transition metal-mediated coupling polymerization, its mechanism is through a typical oxidative addition, transmetalation, and reductive elimination cycle. However, PEDOT prepared through this method showed very low conductivities and is not widely pursued.
PEDOT:PSS is predominantly available as aqueous dispersions (Figure 1c) in the commercial market through oxidative polymerization,20 branded as Clevios™ by Heraeus (Hanau, Germany) or Orgacon™ by Agfa (Mortsel, Belgium).
Despite the huge commercial success of PEDOT:PSS, its colloidal nature also comes
with several critical issues. First, the hydrophobic PEDOT core is surrounded by a
shell of excess hydrophilic PSS with a particle diameter of several tens of nanometers.
It is difficult to form uniform thin films when the film thickness is below the particle
size. Further, it also limits the types of additives that can be introduced to the
system when processing PEDOT:PSS. For example, ionic or basic species will significantly
disrupt colloidal stability and lead to severe aggregation and precipitation. To address
these challenges, intensive studies have focused on developing fully soluble self-doped
PEDOT. Groenendaal and coworkers21 developed an EDOT derivative with a sodium alkylsulfonate side chain that could be
polymerized into conductive conjugated oligomer (1–5 S cm−1). Konradsson and coworkers22 polymerized the same monomer derivative and achieved conductivities of 12 S cm−1. Nonetheless, the limited conductivity values have restricted the practical use of
this approach. Recently, with precise control over the degree of polymerization, Okuzaki
and coworkers17 achieved an exceptionally high inherent electrical conductivity of 1089 S cm−1 of fully soluble, self-doped PEDOT without any additives or post processing treatments
(Figure 1d). In addition to the efforts on improving conductivity, the poor stretchability of
PEDOT:PSS also inspired the structural design and innovative synthesis of several
intrinsically stretchable derivatives. As an example, a block copolymer consisting
of rigid PSS and soft poly(polyethylene glycol methyl ether acrylate) (PPEGMEA) was
synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization,
which simultaneously acts as a stretchable matrix and stabilizer (Figure 1e).18 The PEDOT:PSS-b-PPEGMEA showed reduced Young’s modulus (41 MPa), improved stretchability (128%),
and higher toughness (10.1 MJ m−3) without additional dopants or plasticizers, which greatly outperforms the blended
material without chemistry design. A subsequent study intensively examines the correlation
between structure and mechanical properties. By synthesizing PSS-b-PPEGMEA with varying block ratios, researchers discovered that extending the soft,
insulating PPEGMEA chain enhances energy dissipation, leading to a reduction in elastic
modulus, an increase in toughness, and an improvement in fracture strain.23 Figure 1 | Chemical synthesis of PEDOT:PSS and its derivatives. (a) Synthesis of PEDOT onto PSS
template can form colloidal gel particles in aqueous dispersion and result in films
with PEDOT:PSS-rich (blue) and PSS-rich (gray) phases. The aggregates/crystallites
support enhanced electronic transport. Reproduced with permission from ref 16. (b) Schematic diagram of oxidative polymerization mechanism of PEDOT. Reproduced
with permission from ref 13. (c) Commercial products of PEDOT:PSS in dry pellet form (e.g., ORGACON™ DRY5) and
in aqueous dispersion form (e.g., ORGACON™ ICP 1050). (d) Oxidative polymerization
of a self-doped EDOT monomer derivative bearing a sodium alkylsulfonate side chain
for fully soluble PEDOT in water with a high conductivity. Reproduced with permission
from ref 17. (e) Synthesis of an intrinsically stretchable PEDOT:PSS-b-PPEGMEA through RAFT polymerization. Reproduced with permission from ref 18. (f) Schematic comparison of PEDOT deposition on solid substrate with and without
an adhesion-promoting layer. Mechanism of electrografting through electron transfer
process. Reproduced with permission from ref 19.
In contrast to bulk oxidative polymerization, electrochemical oxidation is particularly useful when using PEDOT as a coating material for bioelectronic applications. By directly applying biases through predefined metal electrodes, EDOT can be selectively oxidized at the electrode-electrolyte interface to form patterned PEDOT coatings. However, challenges arise due to the poor adhesion of PEDOT to metal or other conductive substrates, which compromises long-term stability and functionality. To overcome this, research has focused on tailoring the chemistry of the EDOT monomer. In one study, an amine-functionalized EDOT derivative (EDOT-NH2) was synthesized and electrografted onto various conducting substrates (Figure 1f).19 Through the electron transfer between CH and NH groups, an aminyl radical was generated and facilitated the formation of covalent bonds between PEDOT and the substrate, leading to a great enhancement in layer adhesion. Beyond monomer modification, the idea of engineering an adhesion-promoting layer to form robust anchoring between PEDOT:PSS and substrates has naturally led to substrate modification. One of the approaches involves grafting electrodes with diazonium-based molecules before initiating EDOT polymerization.24 Electrochemical polymerization also has the capability to control the growth of PEDOT:PSS through electrolysis effectively. By imposing pulsed voltage with different amplitudes and duty cycles, complicated polymeric shapes such as solid coatings, branched fractals, and straight fibers can be achieved without a template.25 This advanced and versatile method opens up new possibilities for creating complex conductors and paves the way for the future development of template-free electrodes with customizable shapes.
Selection guidelines of commercially available PEDOT:PSS
Over the years, both Heraeus and Agfa have released a great variety of PEDOT:PSS product series, which primarily reflect different molar ratios of thiophene groups to sulfonic acid groups.26 One key feature of PEDOT:PSS dispersions is that they can be easily formulated through additive blending or postprocessing techniques towards diverse deposition methods and applications. By altering the film morphology,27,28 the conductivity of PEDOT:PSS films can be tuned from 10−5 S cm−1 all the way to 103 S cm−1. In consideration of different coating processes, several commercial-grade PEDOT:PSS dispersions have been developed with optimized viscosities, spanning a broad spectrum from 15 to 40,000 mPa s. In certain organic electronic devices, PEDOT:PSS is utilized as a hole injection layer, where its work function determines the energy barrier for the adjacent functional layer. The typical work function, ranging between 4.8 and 5.4 eV, is optimal for facilitating charge transfer and injection with rapid kinetics. As a result, PEDOT:PSS is commonly employed in optoelectronic devices, acting as an efficient p-type contact layer. Alternatively, Agfa’s Orgacon™ Dry series (Mortsel, Belgium), offered as dry pellets, are designed to be redispersed in water or other polar organic solvents, facilitating the customization of concentration and viscosity. This flexibility greatly expands the potential for developing diverse processing methods, like inkjet printing. It also alleviates the limitations imposed by predetermined solid concentrations or viscosity in aqueous solutions, offering greater freedom in devising strategies to combine PEDOT with other materials for various applications. Typical properties of commercially available PEDOT:PSS dispersions in water are summarized in Table 1.
| Trade Name | Solid Content in Water (w%) | PEDOT:PSS Ratio (w/w) | pH | Work Function (eV) | Surface Electrical Resistance (SER) (Ω/sq) | Major Application(s) | Refs. |
|---|---|---|---|---|---|---|---|
| Clevios™ P | 1.3 | 1:2.5 | 1.9 | 5.2 | >106 (85% VLT) | Antistatic layer in films and hole injection layer of organic electronics | 1 |
| Clevios™ PVP AI 4083 | 1.3–1.7 | 1:6 | 1.0–2.0 | 5.2 | >106 (95% VLT) | Charge transport/injection layer in organic electronics | 29,30 |
| Clevios™ P VPCH 8000 | 2.4–3.0 | 1:20 | 1.0–2.0 | 5.2 | >106 (95% VLT) | Charge transport/injection layer in organic electronics | 31 |
| Clevios™PH 500 | 1.0–1.4 | 1:2.5 | 1.5–2.5 | — | 150 (93% VLT) | Conductive electrode hole injection layer of organic electronics | 32 |
| Clevios™PH 510 | 1.5–1.9 | 1:2.5 | 1.5–2.5 | — | 150 | 15 | |
| Clevios™PH 1000 | 1.0–1.3 | 1:2.5 | 1.5–2.5 | 4.8–5.0 | 100–1000 (93% VLT) | 13,16 | |
| ORGACON™ICP1000 | 1.1–1.3 | — | 1.5–2.5 | — | 380 (90% VLT) | Conductive electrode and antistatic layer in films | 33 |
| ORGACON™ICP1020 | 1.1–1.3 | — | 1.5–2.5 | — | 240 (90% VLT) | ||
| ORGACON™ICP1050 | 1.15–1.25 | — | 1.5–2.5 | — | 120 (90% VLT) | ||
| ORGACON™DRY | — | — | — | — | 450 (85% VLT) | Pellets to be redispersed in polar organic solvents | 34 |
| ORGACON™DRY5 | — | — | — | — | >200 | 35 | |
| ORGACON™S300 | 0.5–1 | — | 2.0–3.5 | — | 425 (90% VLT) | Transparent conductive electrodes applications as alternative to indium tin oxide (ITO) | 36 |
| ORGACON™S315 | 0.5–1 | — | 2.0–3.5 | — | 125 (90% VLT) | ||
| ORGACON™S305plus | 0.5–1 | — | 2.0–3.5 | — | 225 (90% VLT) | ||
| ORGACON™IJ1005 | 0.8 | — | 1.5–2.5 | 5.2–5.4 | 800 (96% VLT) | Hole injection layer of organic electronics and inkjet printing | 37,38 |
| ORGACON™EL-P5015 | 3 | — | — | — | 200 (SER × OD = 18) | Transparent, screen printable conductive ink with >100 um resolution on all kinds of pretreated substrates (poly (ethylene terephthalate), polycarbonate (PC), acrylonitrile butadiene styrene, polyethylene, poly(methyl methacrylate), glass, etc.) | 39 |
| ORGACON™EL-P3165 | 5 | — | — | — | 415 (SER × OD = 11) | ||
| Orgacon™ N-1005 | 1.5 | — | 7.0 | — | >100 (70% VLT) | Tandem solar cell and conductive electrode | 40,41 |
PEDOT dispersions in nonaqueous solvents are also developed to meet the demand of specialized processing methods and application conditions. In scenarios where short drying time or nonpolar solvents are desirable, PEDOT copolymerized with polysulfonic acid in nonpolar solvent can be employed. Formulations with PEDOT:PSS dispersion in alcohol are useful to lower the surface tension between the layer and the underlying substrate. PEDOT-polyethylene glycol (PEG) copolymer, under the trade name of Aedotron™ (Wheat Ridge, Colorado, USA), can be easily dispersed in polar aprotic solvent and enable thin film formation with minimal corrosivity. PEDOT capped with tetramethacrylate (PEDOT-Meth) acts as a reactive oligomer in nonvolatile solvent for reactions with other functional compounds. Typical properties of commercially available PEDOT nonaqueous dispersions are summarized in Table 2.
| Trade Name | Composition | Solid Content (w%) | pH | Surface Electrical Resistance (SER) (Ω/sq) | Major Application(s) | Refs. |
|---|---|---|---|---|---|---|
| Clevios™ HTL Solar 2 | PEDOT:PSS in alcohol | 0.8 | — | 104–106 | Hole injection layer of organic electronics | 42 |
| Aedotron™ | PEDOT/PEG in nitromethane | 1.0 | — | 600–3000 (70–85% VLT) | Antistatic layer in films and hole injection layer of organic electronics | 43 |
| Oligotron™ | PEDOT-tetra methacrylate in propylene carbonate | 0.5 | — | 1–106 | Photocurable ink | 44 |
Typical strategies to enhance the conductivity of PEDOT:PSS
While commercial products offer a good starting point, proper processing could further boost the conductivity of PEDOT:PSS from the following aspects: phase-separated morphology, crystallization of PEDOT, the removal of insulating PSS, and the degree of oxidation in PEDOT.31 From a sample preparation perspective, post-treatment and direct blending with additives are the primary methods.
Post-treatment
Post-treatment agents like strong acids10,45 and volatile organic solvents11 are effective for treating PEDOT:PSS films. Concentrated sulfuric acid (H2SO4) or nitric acid (HNO3) provide protons that convert PSS− into poly(4-styrenesulfonic acid) (PSSH), which can be removed by water. In the meantime,
protons can disrupt the Coulombic attraction between PEDOT+ and PSS− and foster a phase-segregated morphology favorable for high conductivity. For example,
a simple post-treatment with H2SO4 (Figure 2a) can significantly increase the conductivity to 4380 S cm−1 with enhanced lamellar packing and π–π stacking of the PEDOT conjugation planes.10,45 To investigate the role of acids in boosting the conductivity, Müller-Buschbaum and
coworkers48 performed a systematic study by treating PEDOT:PSS with different weak and strong
acids, namely, hydrochloric acid (HCl), formic acid (HCOOH), HNO3, and H2SO4. Selective PSS removal and structural rearrangement of PEDOT-rich domains due to
an enhanced lamellar stacking were identified as the main reasons leading to high
electrical conductivity, which are also linked to the acids’ strength and dielectric
constant.
Figure 2 | Typical strategies to enhance the conductivity of PEDOT:PSS. (a) Schematics of PEDOT:PSS
treated by concentrated H2SO4, reconfiguring from amorphous grains into crystalline nanofibrils via a charge-separated
transition mechanism. X-ray diffraction results showing a more ordered crystalline
structure of PEDOT:PSS treated by increasing concentration of H2SO4. Molecular packing structure of crystalline PEDOT:PSS showing the lattice parameters
of a preferred lamella stacking. Reproduced with permission from ref 10. (b) Schematic of blending D-sorbitol with PEDOT:PSS. Conductivity variation and
S 2p XPS spectra of s-PEDOT:PSS with and without post water rinsing. Reproduced with
permission from ref 46. (c) Schematics of PEDOT:PSS blended with ionic liquids to increase conductivity
and stretchability. Reproduced with permission from ref 47.
Water-miscible volatile solvents like methanol, due to their capability to wash out excess PSS in the film, are also effectively utilized as post-treatment agents. This process results in an increase in the PEDOT/PSS ratio as evidenced by X-ray photoelectron spectroscopy (XPS) and enhanced conductivity from 0.3 to 1015 S cm−1.11 Other alcohols like ethanol- and propanol-treated PEDOT:PSS films showed moderate conductivity enhancement, due to their lower hydrophilicity and dielectric constants compared to methanol. Techniques that combine solution shearing with methanol treatment have been successful in achieving exceptionally high conductivities, reaching 4600 S cm−1.7 Additionally, the optical transparency is maintained, making it well-suited for use as transparent conductors in optoelectronic devices.
Additive blending
Doping is a common way to increase the concentration of mobile charge carriers for enhanced conduction along the polymer backbone and partially neutralizing anionic PSS−. In PEDOT:PSS, secondary doping involves additional dopants beyond the primary p-type doping between PEDOT+ and PSS−. The first discovery that adding polar aprotic organic solvent such as dimethyl sulfoxide (DMSO) could effectively enhance the conductivity of PEDOT films, has led to extensive research into the impact of various additives such as ethylene glycol (EG), d-sorbitol (Figure 2b),46 ionic liquids (Figure 2c),47 and surfactants, as well as the mechanism of conductivity enhancement.49–53 For example, the polar groups in the additives interact with PSS− chains via strong van der Waals forces, altering the morphology by facilitating more efficient PEDOT packing and removal of excessive PSS. Wei et al.54 first demonstrated with direct evidence that the addition of EG to PEDOT:PSS enhances crystallinity and crystal ordering. This finding was confirmed through comprehensive morphology studies and reliable measurements of carrier mobility and doping density. Incorporating biocompatible d-sorbitol into PEDOT:PSS as both a secondary dopant and plasticizer, the modified PEDOT:PSS demonstrated a conductivity exceeding 1000 S cm−1, which was impressively sustained at strains up to 60%. The enhancement in stretchability is attributed to d-sorbitol’s plasticization effect on PSSH chains, disrupting hydrogen bonds and thereby increasing the polymer’s mechanical flexibility. Compared to sorbitol, ionic liquids have higher polarities to interact with PEDOT:PSS, which leads to even better electrical conductivity (3100 S cm−1) and stretchability (>100%).
Transformation of PEDOT:PSS into Various Forms: Fibers, Gels, and Films
To achieve seamless integration and optimal device performance in the biological environment, the biointerfacing material not only needs to have stretchability to maintain conformal contact under dynamic motions but also be compatible to diverse device fabrication techniques. However, pristine PEDOT:PSS, without any post-treatment or additives, typically exhibits a high Young’s modulus of ∼1 GPa and a brittle nature with an elongation at break of >10% strain.55 This inherent rigidity and fragility make it unsuitable for direct application or integration into device fabrication processes. Through chemical approaches, PEDOT:PSS can be effectively transformed into various multidimensional forms, including fibers, gels, and films. Combined with current device fabrication techniques, they open possibilities for creating high-density and high-precision devices with advanced functionalities.
PEDOT:PSS in the fiber form
Recent progress in fiber-based wearable electronics has been geared towards multifunctional features suitable for wearable electronics. Intrinsically conductive PEDOT:PSS, notable for its excellent electrical conductivity and unique aqueous solution dispersion, presents itself as an ideal material for fibers. There are primarily two methods to fabricate PEDOT:PSS fibers. The first one involves direct coating or deposition of PEDOT:PSS onto a fibrous substrate to create composite conductive fibers.56 However, fibers prepared by this method usually display compromised conductivity and mechanical stretchability due to the insulating and rigid nature of fibrous substrates. In the other method, PEDOT:PSS fibers are created via wet-spinning and demonstrate enhanced conductivity and mechanical robustness.57 During the spinning and drawing processes, the shear forces can significantly promote the alignment of PEDOT:PSS chains, thereby elevating the fibers’ overall properties.58 Combining wet-spinning and post-treatment with concentrated H2SO4, Pan et al.59 developed high-performance PEDOT:PSS fibers with an electrical conductivity of 4029.5 S cm−1. However, simultaneous enhancement of both the conductivity and stretchability of these fibers is still a challenge. By adding ionic liquid into the PEDOT:PSS spinning solution and undergoing subsequent H2SO4-immersion-drawing process, Wang and coworkers60 adressed this issue and prepared PEDOT fibers with increased stretchability (27.7% strain) and high conductivity (4288 S cm−1). The flexible and conductive fiber can be further dedoped by polyethyleneimine (PEI) to fabricate an enhancement-mode organic electrochemical transistor (OECT) for more complex electronic devices in textile form.61
PEDOT:PSS in the gel form
Common methods to achieve a mechanically robust PEDOT-based gel involve in situ polymerization of EDOT within an inert gel matrix,62 or blending PEDOT:PSS with gel precursors to form interpenetrating polymer networks.63 For instance, Bao and coworkers55 developed an interpenetrating hydrogel network by initiating in situ polymerization of acrylic acid within a PEDOT:PSS/ionic liquid mixture that was physically pregelled. This method resulted in a gel with a tunable elastic modulus ranging from 10 to 400 kPa, excellent stretchability exceeding 100%, and commendable conductivity above 0.1 S cm−1. However, this approach faces challenges in balancing electrical and mechanical properties since increasing the content of the acrylic acid matrix tends to diminish the gel’s conductivity. Zhao and coworkers64 devised a novel approach for creating a pure PEDOT:PSS hydrogel, eliminating the need for additional components. Their method involves adding DMSO into aqueous PEDOT:PSS solutions, followed by a controlled dry-annealing process and subsequent rehydration. The key to achieving enhanced electrical conductivity (up to 40 S cm−1) lies in the phase separation that occurs during the anisotropic drying. The pure PEDOT:PSS hydrogel also shows an improvement in stretchability (>35%) and Young’s modulus (2 MPa) compared to pristine pure PEDOT:PSS, but it still requires further optimization for real bioelectronic applications. Applying the same dry-annealing and rehydration technique, the conductivity of PEDOT:PSS hydrogel could be further increased by adding ionic liquid as dopant65 or mixing with metal halide solution as Lewis acid for ion exchange.66 Another template-directed in-situ synthesis strategy proposed by Kang and coworkers67 was achieved by postassembly of the PEDOT:PSS fibers in the nanoconfined space of chemically crosslinked polyacrylic acid template network (Figure 3b).
The integration of additional properties like adhesion, stretchability, and antifouling into PEDOT:PSS hydrogels can be achieved through a synergistic combination with secondary materials, each bearing unique characteristics. For instance, Zheng and coworkers68 successfully infiltrated PEDOT:PSS conductive polymers into a zwitterionic network. This innovation led to a hydrogel with ultrahigh stretchability of 4000%–5000%, a low modulus of approximately 0.5 MPa, and robust antifouling properties. The resulting hydrogel was designed into a dual-sensitive strain sensor, offering highly sensitive, reliable, and precise monitoring of a full range of human activities. Zhao et al.69 developed a bicontinuous conducting polymer ink utilizing different solubilities of the electrical phase, PEDOT:PSS, and the mechanical phase, hydrophilic polyurethane (PU). Unlike earlier methods that struggled to balance conductivity and stretchability, this new approach leads to a phase-separated bicontinuous structure that is easy to fine-tune the mechanical properties of the gel independently. This structure adeptly combines mechanical robustness, demonstrated by over 300% strain and 3,000 J m−2 of toughness, with a notable electrical performance, achieving more than 10 S cm−1 of conductivity. This balance ensures that neither the mechanical nor the electrical properties of the gel are compromised. Bao and coworkers35 further extended the application of conductive PEDOT:PSS hydrogels to the field of wound healing where electrical stimulation applied through the conducting adhesive hydrogel can accelerate wound closure and tissue regeneration (Figure 3c). They developed a PEDOT-based hydrogel electrode characterized by its low contact impedance (∼40 ), exceptional toughness, and high stretchability (∼400%). The low impedance is essential for efficient signal transduction, enhancing the bandage’s functionality. The hydrogel also shows switchable adhesion, facilitating gentle detachment, which is particularly beneficial for applications on sensitive wound areas. This temperature-responsive adhesion is achieved by integrating a thermally responsive covalent network of N-isopropylacrylamide (NIPAM), which undergoes heat-induced aggregation of its amphiphilic unit in water. However, to prepare the thermal responsive PEDOT:PSS hydrogel, previous initiation methods present certain challenges: (1) Thermal initiation at high temperature (e.g., 70 °C) is already higher than the phase transition point of NIPAM, leading to severe aggregation of NIPAM; (2) Conventional radical initiators, which include ionic and basic species, i.e., ammonium persulfate and N,N,N′,N′-tetramethylethylenediamine, tend to cause significant coagulation of the colloidal suspension of PEDOT:PSS. To solve the issue, this research developed a new initiation system based on a nonionic redox pair, that is, hydrogen peroxide (H2O2) and ascorbic acid, allowing for rapid and uniform gelation at room temperature, typically within approximately 3 min.
Notably, because of the substantial amount of water in the gel, the conductivity of
PEDOT:PSS gels is generally lower than that of PEDOT:PSS thin films. Despite not matching
the conductivity levels of thin films, PEDOT:PSS gels have, through targeted chemical
design strategies, achieved notable conductivities, reaching up to approximately 247 S
cm−1.67 To date, the development of PEDOT:PSS gels has made several remarkable strides, achieving
tissue-level softness (∼10–400 kPa),55 ultrahigh stretchability (∼4000%–5000% strain),68 and high conductivity (247 S cm−1).67 Figure 3 | Transformation of PEDOT:PSS into fiber, film, and gel forms. (a) Scheme of fabrication
of conductive fiber by fibrillary gelation at interface between PEDOT:PSS solution
and choline-based ionic liquid, and cross-sectional scanning electron microscopy (SEM)
image of the fiber. Reproduced with permission from ref 61. (b) Schematic illustration of the template-directed in-situ synthesis of electrically
conducting hydrogel with postassembly of the PEDOT:PSS fibers in the nanoconfined
space of the chemically crosslinked polyacrylic acid template network. Reproduced
with permission from ref 67. (c) A conductive PEDOT:PSS hydrogel with temperature-responsive switchable tissue
adhesion. Reproduced with permission from ref 35. (d) An intrinsically stretchable topological supramolecular network of PEDOT:PSS
showing high conductivity under large strain. Reproduced with permission from ref 70.
PEDOT:PSS in the film form
In order to fundamentally address the conflicts between the electrical conductivity and stretchability of PEDOT:PSS, researchers have turned their focus towards chemical approaches by blending with additives. For example, ionic liquids are able to soften the polymer chain as plasticizers while weakening the electrostatic interaction between PEDOT:PSS to induce better aggregation for enhanced conductivity. The best performing ionic additive, that is, bis(trifluoromethane) sulfonimide lithium salt, resulted in a PEDOT:PSS thin film exhibiting a measured conductivity of 608 S cm−1 and a maximum strain of 133%. This development presents new opportunities for the field of bioelectronics, as PEDOT:PSS interconnects can now be utilized to connect rigid components on curved objects.47 Although ionic and molecular additives can improve its conductivity and stretchability, after solvent treatment or immersion in aqueous biological environments, the performance of existing PEDOT:PSS films typically drops substantially because the non-crosslinked additives are washed away. A noteworthy advancement in this field is the rational design of a topological supramolecular network, which adeptly navigates the trade-off between conductivity and stretchability (Figure 3d).70 Moving beyond the conventional approach of blending with small molecule or non-crosslinked additives for mechanical benefits, this innovative work employs molecular engineering centered around a supramolecular structure known as polyrotaxane. The distinct mechanically interlocked structure of polyrotaxane affords significant conformational freedom, enhancing stretchability and delaying the onset of cracking. This approach has led to unprecedentedly high conductivity (∼6000 S cm−1) even at large strains (∼100%) after immersion in physiological fluids. Through meticulous chemical design, transformative effect can be achieved where the interaction of various materials results in emergent properties that surpass the individual contributions of each component. This synergy not only enhances existing features but also unveils new functionalities, demonstrating the power of chemistry in material innovation and application diversification.
Processability of PEDOT:PSS for device fabrication
3D printing
While recent advancements in material design have introduced new functionalities for
PEDOT-based gels, the majority of these developments still depend heavily on in situ
gelation techniques. This simple processing method can only yield bulky devices, such
as strain sensors or conductive pads. To fabricate complex bioelectronic systems,
a more sophisticated approach in chemical design is essential. This shift is particularly
necessary to harness advanced manufacturing technologies like 3D printing, which can
open doors to more intricate and multifunctional bioelectronic device production.
Zhao and coworkers71 first designed a viscous conducting PEDOT:PSS ink from a commercial aqueous solution
(Figure 4a). Initially, the solution was stirred and filtered before cryogenic freezing in a
liquid nitrogen bath. The frozen product was subsequently lyophilized for 72 h to
extract PEDOT:PSS nanofibrils. These nanofibrils, at various concentrations, were
then redispersed in a water-DMSO mixed solvent to achieve the optimal viscosity for
printing applications. However, the mechanical property of the printed PEDOT:PSS was
poor, limiting its utility in bioelectronics where both mechanical and electrical
properties are crucial. This challenge was later overcome by the same research team
through the development of a bicontinuous conducting polymer ink with balanced conductivity
and stretchability.69 The design requires a meticulous selection of an ethanol-water binary solvent composition
to facilitate phase-separation. This separation occurred between the hydrophilic PU,
serving as the mechanical phase, and PEDOT:PSS, acting as the electrical phase, opening
avenues towards a variety of advanced fabrication techniques such as spin-coating,
electrospinning, micromolding, and 3D printing. In these works, tailoring PEDOT ink
for 3D printing has involved adjusting rheological properties and fine-tuning electrical
and mechanical attributes, often encountering trade-offs between these optimizations.
To bypass the necessity of rheological adjustments, a liquid-in-liquid strategy has
been recently reported, a new mechanism that offers more freedom in tuning ink composition.72 This technique relies on assembling PEDOT:PSS colloidal particles from the aqueous
phase and polydimethylsiloxane (PDMS) surfactants from the other phase to form an
elastic film at the liquid–liquid interface. This formation effectively traps hydrogel
precursor inks in designed 3D nonequilibrium shapes, setting the stage for subsequent
gelation and/or chemical cross-linking. Remarkably, this method has achieved conductivities
as high as 301 S m−1 with a relatively low PEDOT:PSS concentration of 9 mg mL−1 within two interpenetrating hydrogel networks. Demonstrating practical application
potential, this technique paves the way for fabricating PEDOT:PSS-based electromicrofluidic
devices and customizing near-field communication implantable biochips in the future.
All of these works mark an advancement in the material-to-device journey of conducting
polymer gels. It effectively bridges a crucial gap in fabrication techniques, thereby
unlocking new possibilities for a wide array of more complicated applications.
Figure 4 | Processability of PEDOT:PSS for device fabrication. (a) Design of 3D printable conducting
polymer ink. The smallest feature size is determined by nozzle size (∼20 μm). Reproduced
with permission from ref 71. (b) Schematic illustration of a direct photopatternable PEDOT:PSS with the smallest
feature size down to 2 μm. Reproduced with permission from ref 70.
Photopatterning
While 3D inkjet printing marks a significant advancement, its highest resolution and smallest achievable feature size, approximately 20–100 μm, are constrained by factors such as nozzle size and ink viscosity. To be compatible with advanced semiconductor manufacturing through photolithography, Bao and coworkers70 employed molecular engineering to design a UV-crosslinkable topological supramolecular structure that allows for direct photopatterning of PEDOT:PSS at the cellular scale (Figure 4b). By carefully tuning the chemical orthogonality and surface energy, this method enabled a scalable fabrication of high-density soft electrode array with unprecedented feature sizes down to 2 μm. They further showed that the high-density soft array could map surface electromyography (sEMG) from human subjects and allows stable recording of muscle signals from soft-bodied octopus. The combination of high-precision, low-modulus, and low impedance further allowed localized neuromodulation at previously challenging locations, for example, brainstem, where organ-specific activities could be evoked with single-nucleus precision.
Device Integration and Applications
PEDOT:PSS-based organic electrochemical transistor (OECT)
In the mid-1980s, OECT was prototyped and its behavior was reported to closely resemble
that of a solid-state transistor.73 A common OECT configuration consists of the following components: three metallic
electrodes (source, drain, and gate), an electrolyte in physical contact with the
gate, and a redox active channel (Figure 5a). The choice of PEDOT:PSS as the channel material is of great significance due to
its unique mixed ionic/electronic conductivity. During operation, a voltage bias applied
between the source and drain (VD) drives the current across the organic channel. Simultaneously applying a gate voltage
(VG) allows for control over the amplitude of this current, determining the level of
ionic injection or retraction and the doping state of the organic film.78 Compared to conventional field-effect transistors (FET) where only surface states
are altered by the gate bias, the entire channel material of OECT can be electrochemically
modulated, which gives rise to its exceptionally high transconductance values for
high-gain amplification of sensor signals.78 Figure 5 | Applications of PEDOT:PSS-based OECT as biochemical sensors, neural interfaces, and
neuromorphic devices. (a) Illustration of basic device configuration and working mechanism
of a three-terminal OECT with a PEDOT:PSS channel. Output and transfer curves of OECTs
with crystallized PEDOT:PSS film channel (Crys-P) shows a larger on-state current
and high transconductance than ethylene glycol treated film (EG-P). Reproduced with
permission from ref 74. (b) Optical micrographs of OECT based electrocorticography probe and layouts of
surface channel and electrode. Recording of a bicuculline-induced epileptiform spike
from a transistor (pink) shows far superior signal-to-noise ratio (SNR) than PEDOT:PSS
surface electrode (blue) and Ir-penetrating electrodes (black). Reproduced with permission
from ref 75. (c) Systematic diagram of the FSP-OECT constructed with a carbon fiber electrode
and a PEDOT:PSS-modified channel and its working principle of electrochemical dedoping
in DA sensing. Reproduced with permission from ref 76. (d) Schematics comparing an OPECT with a biological synapse and channel conductance
measurement with light stimulus. Reproduced with permission from ref 77.
Over the past decades, OECTs embraced a myriad of applications including neural interfaces, biochemical sensors, and neuromorphic devices. As neural interfaces, PEDOT-based OECTs have shown superior performance in signal recording. Malliaras and coworkers75 fabricated an OECT-based electrocorticography probe to record in vivo brain activities and displayed superior signal-to-noise ratio due to local amplification compared with surface electrodes (Figure 5b). As biochemical sensors, OECTs serve as high-performing electrochemical signal transducers for the in vivo detection of critical biomarkers, with high spatial resolution due to their facile processing and amplification of electrochemical signals due to the redox-reactive PEDOT:PSS channel. A highly sensitive nitric oxide (NO) sensor for early diagnosis of osteoarthritis was made possible with a PEDOT:PSS channel, gold terminals, poly-5-amino-1-naphthol as the selective membrane on the gate, and SU-8 encapsulation.79 The oxidation of NO molecules on the gate electrode increases the potential at the electrolyte-channel interface, which converts the PEDOT backbone to its neutralized state and reduces the drain current. With an optimized geometry design, the device is able to offer real-time monitoring of NO concentration in vivo, delivering a low response limit of 3 nM. Mao and coworkers76 demonstrated a fast-scanning potential (FSP)-gated configuration based on an OECT (FSP-OECT), an interdigital electrode modified with PEDOT:PSS channel. The applied gate potential with specific waveform selectively induces oxidation of dopamine (DA) at the carbon fiber electrode and controls the electrochemical dedoping process within the PEDOT:PSS channel, thus enabling DA sensing with high selectivity and sensitivity (Figure 5c). Yan and coworkers80 developed portable OECT-based sensors to detections of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG). The gate electrodes of the OECTs are modified with SARS-CoV-2 spike proteins that selectively capture the IgG through the specific antibody-antigen reaction. The positively charged IgG molecules, forming electrical dipoles on gate surfaces, modulate the channel currents of the OECTs. The devices therefore realize label-free IgG detection. Rivnay and coworkers81 proposed a referenced-OECT-based electrochemical aptamer-based sensor (ref-OECT based E-AB). Because of the active amplification of the OECT device through the doping of the PEDOT:PSS channel, a sensing platform for transforming growth factor beta 1 yields a remarkable enhancement in sensitivity, measuring 3–4 orders of magnitude higher than traditional E-AB sensors. Evidently, the ionic-sensitive channel of OECT can serve as a route for tracking cellular dynamics. Hsing and coworkers82 positioned an OECT array alongside a monolayer of epithelial cells, creating a barrier for ion motion in the electrolyte. The PEDOT:PSS channel demonstrates outstanding biocompatibility by supporting a confluent monolayer of Calu-3 cells. Merging the electrochemical characteristics of OECT with transepithelial ion transport, this device creates a framework for quantitative investigation of epithelial cell behavior and drug screening that targets ion transport channels.
Meanwhile, OECTs can be implemented as neuromorphic devices. The conductance state of an OECT can be temporarily or permanently modified through electrochemical doping of the organic channel, and this process mirrors biological synapse functions such as short-term plasticity and long-term plasticity. For example, an electrochemical neuro morphic device was designed to capture the behavior of an artificial synapse via nonvolatile control of the conductivity of an organic mixed ionic/electrical conductor. When a positive presynaptic voltage (Vpre) is applied on the presynaptic PEDOT:PSS electrode and induces cation flowing toward the postsynaptic electrode through the electrolyte, the number of holes is reduced on the PEDOT backbone and causes a decrease in the conductivity of the PEDOT:PSS/poly(ethylenimine) (PEI) electrode. A negative Vpre will reverse the process. This artificial synapse features more than 500 conductance states while operating at a switching voltage of 0.5 mV.83 In follow-up research, a biohybrid artificial synapse was reported to implement the artificial neural network in hardware. Briefly, dopamine released from PC-12 cells are locally oxidized on the PEDOT:PSS gate, and the ionic flow, as demonstrated in the previous work,83 modifies the conductance state of the downstream PEDOT:PSS channel. A PDMS microfluidic channel was deployed to recycle the neurotransmitter and its oxidized product, in emulation of the endocytosis pathway of synapse.84 Ultimately, the device enables direct coupling of the conductance state of the device with the activity of neurotransmitters. Santoro presented an organic photoelectrochemical transistor (OPECT)-based platform including a fully organic light-responsive gate electrode featuring PEDOT:PSS covalently bonded to azobenzenes moieties (azo-tz-PEDOT:PSS) through click-chemistry while maintaining excellent electromechanical stability and biocompatibility. This novel light-responsive PEDOT:PSS creates optoelectronic platforms for mimicking and interfacing biological systems. Its integration as gate electrode in OPECT is capable of biomimicking the vertebrate retina’s visual pathways (Figure 5d).77
Limitations of PEDOT:PSS in OECTs are evident. PEDOT:PSS in its pristine form still has a higher Young’s modulus compared to soft tissues, which necessitates the incorporation of other additives that could potentially compromise its electrical conductivity. The intricate hierarchy of PEDOT:PSS can pose challenges for detailed examination of its structure–function relationship when it interacts with other components. Furthermore, PEDOT:PSS is usually prepared from acidic water-based solutions, making certain deposition methods or substrates unsuitable due to potential degradation or alteration of the PEDOT:PSS thin film. The large size of PSS significantly influences the quantity of PEDOT in the film, which in turn affects its volumetric capacitance. For this reason, redox-active semiconducting polymers,4 such as poly(2-(3,3′-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-[2,2′-bithiophen]-5)yl thiophene) (p(g2T-T)), can exhibit higher transconductance than PEDOT:PSS with the same OECT device geometry. Recent research has demonstrated a p(g2T-T)-based OECT with record high transconductance of 223 S cm−1 under 100% strain.85
PEDOT:PSS-based physical sensors
Strain sensor
The strain levels in different areas of the human body can provide insight into several
physical or physiological factors in correlation to one’s state of health. An example
of this is the measurement of epidermal strain on the wrist, which is important for
monitoring heart rate. Additionally, analyzing strains on the joints of fingers and
limbs can provide valuable insights into the athletic performance and rehabilitation
progress of individuals. PEDOT:PSS has been employed due to its high conductivity
with good bending flexibility. In the realm of strain sensors, pristine PEDOT:PSS
faces challenges due to its brittleness and restricted stretchability. Lipomi and
coworkers86 investigated the effects of common additives (DMSO, Zonyl, and PEI) on the stretchability
of PEDOT:PSS thin film. With 5% DMSO and 10% Zonyl, the PEDOT:PSS formulation was
optimized to act as a piezoresistive strain gauge and tolerate up to 20% strain, suitable
for monitoring the motion of the second knuckles (Figure 6a). An alternative approach to extend the maximal strain of the sensors is to process
PEDOT:PSS into flexible forms such as fibers or gels. Khademhosseini and coworkers89 suggested a strain sensor with elastomer covered by bilayer PEDOT:PSS thin films
can boost the overall performance. Within the layers, microislands are formed through
uniaxial prestretch and release. Stretching the device will slide the overlapping
layers through each other, resulting in an increase in contact resistance and ultimately
a detectable change in resistance. The proposed sensor successfully detected various
human activities that are associated with physical and psychological well beings,
such as phonation, swallowing, and limb movement.
Figure 6 | Applications of PEDOT:PSS-based physical sensing devices as strain sensors, pressure
sensors, and temperature sensors. (a) Photograph of a strain sensor comprising PEDOT:PSS
films with 5% DMSO and 10% Zonyl transferred onto thin PDMS substrates and change
in relative resistance of sensor under applied strain by finger bending. Reproduced
with permission from ref 86. (b) Schematic illustration and SEM image of a pyramidal-shaped pressure sensor array.
Demonstration of blood pressure monitoring using a pressure sensor. Reproduced with
permission from ref 87. (c, d) Image of an e-finger temperature sensor based on inkjet-printed MFSOTE matrix
touching an ice cube and the corresponding temperature and pressure mappings of the
sensing array. Schematic illustrations, photos, and measured data of the integrated
array for simultaneous temperature and pressure monitoring. Reproduced with permission
from ref 88.
Pressure sensor
PEDOT:PSS can be easily deposited as a thin film coating onto flexible substrates or 3D surfaces, enabling the fabrication of conformal and flexible pressure sensors. Chung and coworkers87 suggested a micropatterned pyramid PDMS array, whose high shape factor allows extensive deformation under pressure, coated with PEDOT:PSS/PU as the conductive electrode excels with the capacity to detect the pressure of a 93 mg object (23 Pa) at 40% stretch. Such an outstanding sensitivity enables noninvasive blood pressure monitoring when positioning the sensor on a radial artery (Figure 6b). Cutting-edge design of physical sensors permits the use of microstructure-supported organic thermoelastic (MFSOTE) materials for dual-parameter physical sensing. Once the material encounters an object with combined temperature and pressure stimuli, it can use the thermoelectric effect to detect the temperature difference and generate a corresponding voltage. The microstructure frame, in the meantime, undergoes deformation due to an external force, leading to a change in the resistance of the active layer depending on the applied pressure. As a result, both temperature and pressure stimuli can be individually measured. For the demonstration, an inkjet-printed MFSOTE array of 2 × 3 cm2 area with 1350 pixels was fabricated, showing a capacity of collecting spatially resolved pressure (2–3 kPa) and temperature (0–5 °C) information when in contact with an ice cube. The characteristics of MFSOTEs suggest their potential for use in skin-like intelligent devices (Figure 6c,d).88
Temperature sensor
Human body temperature is a crucial indicator of physiological functions. Monitoring an individual’s body temperature can offer valuable information about his or her overall health by enabling cardiovascular and pulmonary diagnosis and the prediction of related syndromes. The effectiveness of PEDOT:PSS as a potential material for wearable temperature sensors has been established, owing to its favorable mechanical and electrical properties, as well as its suitability for simple, patternable, and highly reproducible fabrication methods like printing and spin-coating. Fundamentally, the hygroscopic property of PEDOT:PSS allows for superior thermal responses: temperature fluctuation can greatly affect the water content in the PEDOT:PSS material and subsequently induce microstructure changes. At room temperature, water molecules are absorbed into the material by forming hydrogen bonds with the sulfonic acid groups on PSS. The effective thickness of the insulating PSS shell surrounding the conducting PEDOT core is magnified, resulting in an elevated barrier for electron tunneling and an increase in resistance. This effect is attenuated when the PSS shell loses water at higher temperature. The intricate interplay between temperature and PEDOT:PSS microstructure is the key factor for a highly sensitive temperature sensor. Tokito and coworkers90 reported a printable, stable temperature sensor that harnesses the hygroscopicity of PEDOT:PSS. The device introduces (3-glycidyloxypropyl)trimethoxysilane, a crosslinker for PSS, to the PEDOT:PSS thin film and the fluorinated polymer passivation (CYTOP™, Exton, PA, USA) atop to exclude moisture from the ambient environment, achieving a sensitivity −0.77% °C−1 when the relative humidity changes from 30% to 70%, an ambient stability which is rarely achieved by flexible temperature sensors and thus enable reliable breathing rate and pattern monitoring. Wang and coworkers91 fabricated a PEDOT:PSS sensing layer with microcracks on a PDMS substrate through prestretch and H2SO4 treatment. The swelling of the PDMS substrate at temperatures widens the microcracks, leading to an increase in resistance and change in electrical readout. The fabricated sensor provides a combined high temperature sensitivity of 0.042 °C–1 with an excellent linearity of 0.998 around body temperature, resulting in accurate on-skin temperature readouts for personal healthcare.
Electrochromic display
By incorporating PEDOT:PSS into an electronic system, it can function as part of an electrochromic display (ECD) to visualize the data gathered by linked sensors. In contrast to traditional inorganic electrochromic materials burdened by high cost and low flexibility, organic electrochromic materials are solution-processable and compatible with the fabrication of flexible and large-area devices at low costs. PEDOT:PSS, which undergoes coloration or bleaching through oxidation–reduction reactions, possesses electrochromic properties while acting as an electrode. Therefore, it is possible to organize the electrode and electrochromic layers (or ion storage layer) into a single layer.92 In a sweat sensing platform devised by Wang and coworkers,93 PEDOT-based ECD provides a visual presentation of the analyte concentration. In the sensing platform, two potentiometric sensors and another amperometric sensor record pH alongside with the concentrations of sodium, glucose, and lactose. The outputs are processed by a microcontroller unit that accordingly controls the redox states of 10 PEDOT:PSS ECD pixels and thus their colors. This integrated stretchable sweat sensor enables wiring-free data visualization in real time at low power consumption, thus rendering it an effective on-body health monitoring platform. Bao and coworkers94 reported an intrinsically stretchable diode rectifying a maximum signal frequency of 13.56 MHz under 50% strain. When integrated with a carbon nanotube-based strain sensor and a flexible power circuit, the ECD, composed of two PEDOT:PSS layers with an electrolyte in junction, can visualize the resistance change of the circuit and therefore the applied strain. This design realized an on-skin wireless stretchable system to visualize signals from a sensor by a display pixel, which is expected to aid in the development of wireless and high-speed personal healthcare systems that emulate skin.
PEDOT:PSS for electrophysiological sensing
Spatiotemporal fluctuations of ionic levels play a key role in transmitting signals
between electrogenic cells in the nervous system or muscular tissue. Therefore, understanding
the activity of individual neurons and network functions, as well as diagnosing diseases
at the point of care, heavily relies on accurate recording of the electrophysiological
signals. In both in vitro and in vivo settings, microscale extracellular electrodes
are the key players for simultaneous, multiplexed, and long-term electrophysiological
recordings. The physical mechanisms of extracellular sensors are quantitatively characterized
by equivalent circuit models.95 Efficient and stable signal transduction within this framework entails electrodes
with low interfacial impedance or, equivalently, high areal or volumetric capacitance.96 Compared to inorganic gold electrodes, the volumetric conduction of PEDOT:PSS leads
to significantly decreased interfacial impedance (Figure 7a, top panel), which is essential to achieving high signal-to-noise ratio of the recorded
data.
Figure 7 | Applications of PEDOT:PSS as electrophysiological sensors. (a) Impedance per unit
area of PEDOT:PSS compared with Au and interfacial capacitance per unit area from
PEDOT:PSS and Au as a function of electrode thickness. PEDOT:PSS shows significantly
reduced impedance than Au due to its thickness dependent volumetric capacitance. Exploded
view and an optical microscope image of the active site of the stretchable electrode
array for sEMG measurement. Tracking EMG signals across different channels resolves
the spatiotemporal signal propagation of hand gestures. Reproduced with permission
from ref 70. (b) Schematics and photos showing the implantation process of soft PEDOT electrodes.
Stable enclosure was achieved by wrapping soft PEDOT electrodes around the sciatic
nerve and pressing gently. Illustration of soft PEDOT electrodes wrapped around the
facial–acoustic nerve complex showing multiplexed intraoperative monitoring during
vestibular schwannoma (VS) surgery in a rabbit model. Reproduced with permission from
ref 97. (c) Schematic illustration showing the relative position of the elastic electrode
array on the surface of a rabbit heart and optical photographic image demonstrating
the freestanding elastic electrode array conforming to the surface of an ex vivo rabbit
heart. Spatiotemporal epicardial-potential data capturing of cardiac activation propagating
across the array. Reproduced with permission from ref 98. (d) The NeuroGrid. Intraoperative photograph showing 240-channel NeuroGrid (yellow
circle) conforming to the surface of the cortex. Examples of the spatial extent and
normalized neural firing rate of extracellular action potentials over the geometry
of the array. Reproduced with permission from ref 99.
Malliaras and coworkers100 reported a novel fabrication process for PEDOT:PSS-based microelectrode arrays (MEA). At 1 kHz, the array achieved an impedance of 23 kΩ, a value decimated from that of an MEA with gold electrodes (400 kΩ). Through a molecular engineering strategy, Bao and coworkers70 designed a topological supramolecular network based on a polyrotaxane structure where a single conducting polymer system achieves exceptional conductivity, stretchability, and photopatternability. The resulting soft electrode array allowed for high-density sEMG recording with over one order of magnitude reduction of inter-electrode distance compared to commercial products (Figure 7a).
Based on the same supramolecular conducting polymer network, soft PEDOT:PSS electrodes also allowed for robust contacts with peripheral nerves. Even under tugging events during neurosurgery, PEDOT:PSS electrodes could still record stable nerve signals, therefore enabling continuous intraoperative neurophysiological monitoring (CINM) whereas clinically-used ballpoint electrodes can only provide intermittent signals. By wrapping around different nerves, the electrode also displays multiplexing capability in distinguishing adjacent nerves. Hence, appropriate identification of nerves during clinical procedures became a possibility (Figure 7b).97 With the capability of CINM, enhanced postoperative prognosis could be achieved by alerting nonintentional nerve damages during surgery. Further, PEDOT:PSS-based electrode arrays can also operate on fast-beating heart tissue. On rabbits with heart rates in the range of 120 to 150 beats per minute, the soft electrode array could still provide stable in vivo recording over cyclical stretching (Figure 7c).98,101 For the central nervous system, Buzsáki et al.102 developed a PEDOT:PSS-based neural interface array (NeuroGrid) that can conform to the rat somatosensory cortex. Due to the reduced interfacial impedance, a low noise, highly stable recording of single unit action potentials even from distal sites became possible. To cover a larger spatial area with the goal of monitoring the initiation and propagation of physiological activities in the human brain, Buzsáki and coworkers99 designed NeuroGrids with 120 and 240 electrodes to cover 420 or 840 mm2 of neocortex, respectively (Figure 7d).
Notably, OECT offers a unique opportunity for precise electrophysiological monitoring through the PEDOT:PSS channel’s volumetric capacitance and the absence of a physical barrier between the organic channel and electrolyte. The first in vivo use of brain activity recording was empowered by a highly conformable OECT array. Due to the local amplification of signal endowed by the transistor circuit, the OECT array obtains a signal-to-noise ratio of 22.3 dB, outperforming PEDOT:PSS surface electrodes (13.5 dB) and penetrating silicon electrodes (18.2 dB) in the rat model. In other words, the PEDOT:PSS OECTs are prone to collect small and local signals, which could empower precise identification of zones of microseizures, a vital diagnostic process in the field of epilepsy.75 Khodagholy and coworkers103 created an internal ion-gated transistor (IGT) by utilizing PEDOT:PSS and D-sorbitol to incorporate mobile ions into the organic channel. An additional chitosan layer was introduced to enable ionic, rather than electrical, conduction between the gate and the channel. This device architecture facilitates ionic exchange through the individual organic channel in each IGT, rather than relying on a shared electrolyte. In the end, an IGT array possesses a remarkable transconductance of 32 mS and an ultralow time constant of 2.6 μs, making it highly capable of capturing single unit action potential signals.
PEDOT:PSS for electrical stimulation
The use of electrical stimulation allows for precise manipulation of biological processes, promotes interaction between electronic devices and living systems, and presents potential treatments for a range of medical conditions and biomedical obstacles. Stimulation and recording of biological tissues are governed by the same physical principles, although proceeding in the opposite direction. To facilitate efficient electrical stimulation, capacitive charge injection based on (dis)charging of the electrolyte–electrode interface, as elucidated in equivalent circuit models, is preferred over faradaic charge injection, in which the high potential at the electrode is highly associative with electrode degradation, water hydrolysis, and tissue damage.104 For comprehensive investigations of the behavior of electrical active tissues, small-size and high-density stimulation devices are required to match the physical dimension of the targeted cells.105 The key to overcoming the impedance at this dimension is to use electrodes with high charge injection capability. PEDOT:PSS, acting as both a capacitor and a conductor, is well-suited for this role.
Utilizing the aforementioned supramolecular network with superior charge storage/injection
capacity, Bao and coworkers70 demonstrated a stretchable electrode array for precise neuromodulation through intimate
contact with the brain tissue and, therefore, obtain high-resolution activation maps
that correlated with individual nuclei for hypoglossal, facial, and accessory nerves
that innervated, respectively, the genioglossus for the tongue, the orbicularis oris
for the whisker, and the sternocleidomastoid for the neck (Figure 8a). A PEDOT:PSS-based implantable neural cuff electrode has also been extensively studied.
In another work, Bao and coworkers107 fabricated a soft and elastic hydrogel-based microelectronic device by exchanging
the ionic liquid additive in PEDOT:PSS with water and employing an elastic fluorinated
photoresist as the passivation layer. The micropatterned conductive hydrogel electrode
achieved a Young’s modulus of 32 kPa, matching the mechanical behavior of nerve, leading
to minimal inflammatory tissue in mouse model. The hydrogel electrode was able to
maintain its performance in eliciting a leg response in rat models even after being
immersed in phosphate buffered saline (PBS) for 2 months. Furthermore, it was able
to evoke muscle twitching at a low stimulation threshold of 50 mV, which is only a
tenth of the voltage needed for Pt electrodes.
Figure 8 | PEDOT:PSS for electrical stimulation. (a) Charge storage/injection capacity of PEDOT:PSS
in comparison with Au electrode. Schematic diagram illustrating the application of
the stretchable electrode array for precise neuromodulation through localized brainstem
stimulation and microscopic image of a stretchable electrode array conforming to the
curved floor of the fourth ventricle with activation maps depicting the spatial distribution
of different nuclei in the brainstem. Reproduced with permission from ref 70. (b) Image showing the components of a monolithically integrated, soft e-skin patch
and schematic diagram of the structure of the artificial sensorimotor system. Pulse-train
output frequencies and physical response in a rat model under different applied pressure
levels. Reproduced with permission from ref 106. (c) Schematic diagram and exploded view of a wireless smart bandage including flexible
printed circuit board (FPCB) and tissue-interfacing conducting adhesive hydrogel.
IR image of a mouse wearing the smart bandage and raw traces of wirelessly sensed
temperature and impedance. Representative photographs showing the progression of wound
regeneration with and without electrical stimulation treatment. Reproduced with permission
from ref 35.
Integrated circuits are frequently implemented to deliver electrical stimulation to emulate the functionality of skin. Bao et al.106 designed a signal conditioning circuit system on a skin-like patch to generate nerve-like pulse-trains. This patch can simulate the natural sensory process with a driving voltage of less than ±5 V. In addition, carbon nanotube-based stretchable pressure sensors and thin film temperature sensors with three-dimensional pyramid structures were developed and integrated into the system to simulate biological mechanoreceptors and temperature receptors, respectively. To complete the sensorimotor loop, a synaptic transistor with PEDOT:PSS electrode and a single-ion conductive polyelectrolyte were incorporated. Under the exposure to varying levels of forces, the digitalized inputs for the somatosensory cortex evoked a response at the motor cortex and thus downstream muscle actuation (Figure 8b).
Besides stimulating excitable tissues, Bao and coworkers35 applied an interpenetrating hydrogel network of NIPAM and acrylamide, along with physically crosslinked PEDOT:PSS, as the conductive interface in a smart wound management system that offers wireless, closed-looped monitoring and stimulation of the wound site. In a preclinical wound model, the temperature and impedance sensors synergistically provided continuous monitoring of the wound site as the mice roamed in the cage, while the electrical stimulation accelerated wound healing and tissue regeneration (Figure 8c).35
Outlook
This review aims to offer an overview of recent progress in PEDOT:PSS-based bioelectronics. PEDOT:PSS is advantageous considering its electrical properties that combine ionic and electrical dual conduction, its processibility enabling compliant mechanical properties, and its high volumetric capacitance well suited for electrophysiological recording and stimulation. These features substantially enhance the performance of devices in a full array of applications.
Looking ahead, the future of PEDOT:PSS in material design and device applications is poised at a crucial juncture, facing several challenges and opportunities. A primary issue is the trade-off often observed in conducting polymer-based hydrogels, where achieving desirable mechanical properties frequently comes at the cost of reduced electronic performance. This balance between mechanical flexibility and electrical conductivity remains a key area for further research and innovation. Another significant challenge lies in the patternability of PEDOT:PSS materials. The processing and fabrication methods of PEDOT:PSS gels, often rooted in bulk synthesis, limit their application in creating high-density and small-scale features. The ability to pattern these materials with high precision and density is essential for the development of advanced bioelectronic devices, where intricate designs and miniaturization are often required. Developing methods to achieve such fine patterning without compromising the material’s inherent properties is crucial for expanding the scope of PEDOT:PSS in sophisticated device applications. Overcoming this limitation is essential for the material to find broader applicability, particularly in the field of wearable electronics and bio-integrated devices.
In OECT, in which a barrierless ionic exchange between the electrolyte and the organic channel is enabled, it exhibits excellent transconductance and reversible electrochemical properties for high performance biosensors and neuromorphic devices. Regarding the physical sensors, PEDOT-based conductive materials effectively resolve the inherent risk of brittle failure in traditional, metallic conductive materials and seamlessly integrate with biological systems, thus offering great potential in wearable smart devices. Bioelectronics for electrophysiological recording and stimulation fully exploits the low impedance and high charge injection capability of the PEDOT:PSS interface. Exceptional tissue conformality and efficient ionic/electrical signal transduction of PEDOT:PSS based devices have been validated in recent studies.
Although the device physics of PEDOT:PSS-based OECTs is quantitatively described by the Bernards model, utilizing advanced simulations and characterization techniques is necessary to improve our understanding of the molecular behavior during device operation. For example, the most recent technique, electrochemical strain microscopy, is expected to be combined with computational modeling to gain a more thorough understanding of ion flow in the channel, through direct observation of ionic transport.
Physical sensors, which rely on cyclical and robust operation, face a disadvantage due to the susceptibility of PEDOT:PSS to various environmental factors, such as humidity, temperature, and unexpected strain. Physical sensors utilizing PEDOT:PSS also encounter an obstacle in achieving consistent uniformity of the sensing layers, including solid films or gels, during device fabrication. Future studies will be spurred by these obstacles to explore packaging and microprocessing techniques that can be used with PEDOT:PSS physical sensors.
There is still room for progress in addressing challenges related to PEDOT:PSS-based bioelectronics for electrophysiological sensing and modulation. Despite the use of cutting-edge sensors for extracellular potential recordings, they still fall short in detecting subthreshold signals, such as excitatory and inhibitory synaptic potentials that are critical for unveiling neural network dynamics and psychiatric disorders. In addition, what remains underexplored is the direct correlation between the electrophysiological stimulation, or recording, and the synaptic activities of the biological tissue. Consequently, a long lasting, scalable platform for the monitoring of electrophysiological signals and biological activities from neurons holds great importance. The overarching challenges with the PEDOT:PSS bioelectronics are worth noting. The technological development of PEDOT:PSS-based devices is in its early stage when compared to other types of organic electronics, namely organic light-emitting diode, organic FET, and organic photovoltaics. Ongoing research endeavors are encouraged to benchmark device metrics in PEDOT:PSS bioelectronics, including the scalability of fabrication, cyclic stability, and environmental stability. This will facilitate a structured framework for the integration of PEDOT:PSS devices, in contrast to sporadic proof-of-concept investigations, with system-level designs that can be easily adapted to various technologies and applications. Moving onward, the incorporation of bioelectronic sensors alongside state-of-the-art device components like photovoltaic textiles, supercapacitors, and triboelectric generators, brings forth possibilities for the development of sustainable wearable electronics that can detect and analyze electrophysiological and biochemical signals. Additionally, due to the inherent molecular limitations of PEDOT:PSS, it inevitably faces trade-offs in its conductivity, biocompatibility, and stability. Recent developments in materials like conducting hydrogels, n-type materials, and wet tissue adhesives, along with the use of PEDOT:PSS in upcoming bioelectronic devices, present viable options to enhance mechanical performance and biocompatibility while also expanding electrochemical properties.
In the future, advancing PEDOT:PSS’s applications will likely involve a multidisciplinary approach, combining insights from materials science, chemistry, device engineering, and medical applications. Emphasis on developing new chemical designs and processing techniques that can simultaneously enhance conductivity, stretchability, and long-term stability will be pivotal. As research continues to push the boundaries, PEDOT:PSS is expected to play a transformative role in the next generation of bioelectronic devices, offering potential solutions to longstanding challenges in the field.
Author Contributions
Y.H. and L.T. wrote the manuscript. Y.J. supervised the work and edited the manuscript.
Conflicts of Interests
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the start-up funding from the University of Pennsylvania.
References
- 1. Jeong J.-W.; Shin G.; Park S. I.; Yu K. J.; Xu L.; Rogers J. A.Soft Materials in Neuroengineering for Hard Problems in Neuroscience.Neuron2015, 86, 175–186. Google Scholar
- 2. Jiang Y.; Tian B.Inorganic Semiconductor Biointerfaces.Nat. Rev. Mater.2018, 3, 473–490. Google Scholar
- 3. Ganji M.; Tanaka A.; Gilja V.; Halgren E.; Dayeh S. A.Scaling Effects on the Electrochemical Stimulation Performance of Au, Pt, and PEDOT:PSS Electrocorticography Arrays.Adv. Funct. Mater.2017, 27, 1703019. Google Scholar
- 4. Paulsen B. D.; Tybrandt K.; Stavrinidou E.; Rivnay J.Organic Mixed Ionic–Electronic Conductors.Nat. Mater.2020, 19, 13–26. Google Scholar
- 5. Oldroyd P.; Gurke J.; Malliaras G. G.Stability of Thin Film Neuromodulation Electrodes Under Accelerated Aging Conditions.Adv. Funct. Mater.2023, 33, 2208881. Google Scholar
- 6. Toušek J.; Rutsch R.; Toušková J.Explanation of the High Conductivity of HCl Protonated Polyaniline Films.Mater. Chem. Phys.2021, 260, 124153. Google Scholar
- 7. Worfolk B. J.; Andrews S. C.; Park S.; Reinspach J.; Liu N.; Toney M. F.; Mannsfeld S. C. B.; Bao Z.Ultrahigh Electrical Conductivity in Solution-Sheared Polymeric Transparent Films.Proc. Natl. Acad. Sci.2015, 112, 14138–14143. Google Scholar
- 8. Pang A. L.; Arsad A.; Ahmadipour M.Synthesis and Factor Affecting on the Conductivity of Polypyrrole: A Short Review.Polym. Adv. Technol.2021, 32, 1428–1454. Google Scholar
- 9. Shi H.; Liu C.; Jiang Q.; Xu J.Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review.Adv. Electron. Mater.2015, 1, 1500017. Google Scholar
- 10. Kim N.; Kee S.; Lee S. H.; Lee B. H.; Kahng Y. H.; Jo Y.; Kim B.; Lee K.Highly Conductive PEDOT:PSS Nanofibrils Induced by Solution-Processed Crystallization.Adv. Mater.2014, 26, 2268–2272. Google Scholar
- 11. Alemu D.; Wei H.-Y.; Ho K.-C.; Chu C.-W.Highly Conductive PEDOT:PSS Electrode by Simple Film Treatment with Methanol for ITO-Free Polymer Solar Cells.Energy Environ. Sci.2012, 5, 9662. Google Scholar
- 12. Winther-Jensen B.; Breiby D. W.; West K.Base Inhibited Oxidative Polymerization of 3,4-Ethylenedioxythiophene with Iron(III)Tosylate.Synth. Met.2005, 152, 1–4. Google Scholar
- 13. Horii T.; Hikawa H.; Mochizuki Y.; Okuzaki H.Synthesis and Characterization of Highly Conductive PEDOT/PSS Colloidal Gels.Trans. Mater. Res. Soc. Jpn.2012, 37, 515–518. Google Scholar
- 14. Jiang Y.; Liu T.; Zhou Y.Recent Advances of Synthesis, Properties, Film Fabrication Methods, Modifications of Poly(3,4-ethylenedioxythiophene), and Applications in Solution-Processed Photovoltaics.Adv. Funct. Mater.2020, 30, 2006213. Google Scholar
- 15. Jonas F.; Heywang G.; Schmidtberg W.; Heinze J.; Dietrich M.Polythiophenes, Process for Their Preparation and Their Use. US4959430A, September 25, 1990. https://patents.google.com/patent/US4959430A/en (accessed February 28, 2024). Google Scholar
- 16. Rivnay J.; Inal S.; Collins B. A.; Sessolo M.; Stavrinidou E.; Strakosas X.; Tassone C.; Delongchamp D. M.; Malliaras G. G.Structural Control of Mixed Ionic and Electronic Transport in Conducting Polymers.Nat. Commun.2016, 7, 11287. Google Scholar
- 17. Yano H.; Kudo K.; Marumo K.; Okuzaki H.Fully Soluble Self-Doped Poly(3,4-Ethylenedioxythiophene) with an Electrical Conductivity Greater than 1000 S Cm −1.Sci. Adv.2019, 5, eaav9492. Google Scholar
- 18. Kayser L. V.; Russell M. D.; Rodriquez D.; Abuhamdieh S. N.; Dhong C.; Khan S.; Stein A. N.; Ramírez J.; Lipomi D. J.RAFT Polymerization of an Intrinsically Stretchable Water-Soluble Block Copolymer Scaffold for PEDOT.Chem. Mater.2018, 30, 4459–4468. Google Scholar
- 19. Ouyang L.; Wei B.; Kuo C.; Pathak S.; Farrell B.; Martin D. C.Enhanced PEDOT Adhesion on Solid Substrates with Electrografted P(EDOT-NH2).Sci. Adv.2017, 3, e1600448. Google Scholar
- 20. Jonas F.; Krafft W.; Muys B.Poly(3,4-ethylenedioxythiophene): Conductive Coatings, Technical Applications and Properties.Macromol. Symp.1995, 100, 169–173. Google Scholar
- 21. Zotti G.; Zecchin S.; Schiavon G.; Groenendaal L. B.Electrochemical and Chemical Synthesis and Characterization of Sulfonated Poly(3,4-Ethylenedioxythiophene): A Novel Water-Soluble and Highly Conductive Conjugated Oligomer.Macromol. Chem. Phys.2002, 203, 1958–1964. Google Scholar
- 22. Lang U.; Müller E.; Naujoks N.; Dual J.Microscopical Investigations of PEDOT:PSS Thin Films.Adv. Funct. Mater.2009, 19, 1215–1220. Google Scholar
- 23. Blau R.; Chen A. X.; Polat B.; Becerra L. L.; Runser R.; Zamanimeymian B.; Choudhary K.; Lipomi D. J.Intrinsically Stretchable Block Copolymer Based on PEDOT:PSS for Improved Performance in Bioelectronic Applications.ACS Appl. Mater. Interfaces2022, 14, 4823–4835. Google Scholar
- 24. Chhin D.; Polcari D.; Guen C. B.-L.; Tomasello G.; Cicoira F.; Schougaard S. B.Diazonium-Based Anchoring of PEDOT on Pt/Ir Electrodes via Diazonium Chemistry.J. Electrochem. Soc.2018, 165, G3066–G3070. Google Scholar
- 25. Eickenscheidt M.; Singler E.; Stieglitz T.Pulsed Electropolymerization of PEDOT Enabling Controlled Branching.Polym. J.2019, 51, 1029–1036. Google Scholar
- 26. Lövenich W.PEDOT-Properties and Applications.Polym. Sci. Ser. C2014, 56, 135–143. Google Scholar
- 27. Lang U.; Naujoks N.; Dual J.Mechanical Characterization of PEDOT:PSS Thin Films.Synth. Met.2009, 159, 473–479. Google Scholar
- 28. Sakkopoulos S.; Vitoratos E.Differentiation of the Aging Process of PEDOT:PSS Films under Inert Helium and Ambient Atmosphere for Two Different Rates of Thermal Treatment.Open J. Org. Polym. Mater.2014, 4, 1–5. Google Scholar
- 29. Borazan I.; Bedeloğlu A. C.; Demir A.A Comparative Approach to Enhance the Electrical Performance of PEDOT:PSS as Transparent Electrode for Organic Solar Cells.Polym. Polym. Compos.2020, 28, 66–73. Google Scholar
- 30. Woon K. L.; Wong W. S.; Chanlek N.; Nakajima H.; Tunmee S.; Lee V. S.; Ariffin A.; Songsiriritthigul P.Work Function Modification of PEDOT:PSS by Mixing with Barium Acetylacetonate.RSC Adv.2020, 10, 17673–17680. Google Scholar
- 31. Fan X.; Nie W.; Tsai H.; Wang N.; Huang H.; Cheng Y.; Wen R.; Ma L.; Yan F.; Xia Y.PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications.Adv. Sci.2019, 6, 1900813. Google Scholar
- 32. Lee J. J.; Lee S. H.; Kim F. S.; Choi H. H.; Kim J. H.Simultaneous Enhancement of the Efficiency and Stability of Organic Solar Cells Using PEDOT:PSS Grafted with a PEGME Buffer Layer.Org. Electron.2015, 26, 191–199. Google Scholar
- 33. Karalis G.; Tzounis L.; Mytafides C. K.; Tsirka K.; Formanek P.; Stylianakis M.; Kymakis E.; Paipetis A. S.A High Performance Flexible and Robust Printed Thermoelectric Generator Based on Hybridized Te Nanowires with PEDOT:PSS.Appl. Energy2021, 294, 117004. Google Scholar
- 34. Wagner M.; O’Connell C. D.; Harman D. G.; Sullivan R.; Ivaska A.; Higgins M. J.; Wallace G. G.Synthesis and Optimization of PEDOT:PSS Based Ink for Printing Nanoarrays Using Dip-Pen Nanolithography.Synth. Met.2013, 181, 64–71. Google Scholar
- 35. Jiang Y.; Trotsyuk A. A.; Niu S.; Henn D.; Chen K.; Shih C.-C.; Larson M. R.; Mermin-Bunnell A. M.; Mittal S.; Lai J.-C.; Saberi A.; Beard E.; Jing S.; Zhong D.; Steele S. R.; Sun K.; Jain T.; Zhao E.; Neimeth C. R.; Viana W. G.; Tang J.; Sivaraj D.; Padmanabhan J.; Rodrigues M.; Perrault D. P.; Chattopadhyay A.; Maan Z. N.; Leeolou M. C.; Bonham C. A.; Kwon S. H.; Kussie H. C.; Fischer K. S.; Gurusankar G.; Liang K.; Zhang K.; Nag R.; Snyder M. P.; Januszyk M.; Gurtner G. C.; Bao Z.Wireless, Closed-Loop, Smart Bandage with Integrated Sensors and Stimulators for Advanced Wound Care and Accelerated Healing.Nat. Biotechnol.2023, 41, 652–662. Google Scholar
- 36. Boratto M. H.; Nozella N. L.; Ramos R. A.; Silva R. A. D.; Graeff C. F. O.Flexible Conductive Blend of Natural Rubber Latex with PEDOT:PSS.APL Mater.2020, 8, 121107. Google Scholar
- 37. Kim H. G.; Kim M.; Kim S. S.; Paek S. H.; Kim Y. C.Silver Nanowire/PEDOT:PSS Hybrid Electrode for Flexible Organic Light-Emitting Diodes.J. Sci. Adv. Mater. Devices2021, 6, 372–378. Google Scholar
- 38. Buga C.; Viana J.Optimization of Print Quality of Inkjet Printed PEDOT:PSS Patterns.Flex. Print. Electron.2022, 7, 045004. Google Scholar
- 39. Futsch R.; Mjejri I.; Rakotozafy H.; Rougier A.PEDOT:PSS-V2O5 Hybrid for Color Adjustement in Electrochromic Systems.Front. Mater.2020, 7, 78. Google Scholar
- 40. Zakharko Y.; Held M.; Graf A.; Rödlmeier T.; Eckstein R.; Hernandez-Sosa G.; Hähnlein B.; Pezoldt J.; Zaumseil J.Multispectral Electroluminescence Enhancement of Single-Walled Carbon Nanotubes Coupled to Periodic Nanodisk Arrays.Opt. Express2017, 25, 18092. Google Scholar
- 41. Fthenakis V., Ed. Third Generation Photovoltaics; InTech: London, 2012. Google Scholar
- 42. Schumann S.; Elschner A.; Gaiser D.; Lövenich W.Non-Aqueous PEDOT:PSS Dispersion for Improved Inverted Organic Solar Cells.MRS Proc.2015, 1771, 207–212. Google Scholar
- 43. Song I.-S.; Heo S.-W.; Lee J.; Moon D.-K.Study on the ClO4 Doped PEDOT-PEG in Organic Solvent Using a Hole Injection Layer for PLEDs.J. Ind. Eng. Chem.2011, 17, 651–656. Google Scholar
- 44. He J.; Su J.; Wang J.; Zhang L.Synthesis of Water-Free PEDOT with Polyvinylpyrrolidone Stabilizer in Organic Dispersant System.Org. Electron.2018, 53, 117–126. Google Scholar
- 45. Yeon C.; Yun S. J.; Kim J.; Lim J. W.PEDOT:PSS Films with Greatly Enhanced Conductivity via Nitric Acid Treatment at Room Temperature and Their Application as Pt/TCO-Free Counter Electrodes in Dye-Sensitized Solar Cells.Adv. Electron. Mater.2015, 1, 1500121. Google Scholar
- 46. He H.; Zhang L.; Guan X.; Cheng H.; Liu X.; Yu S.; Wei J.; Ouyang J.Biocompatible Conductive Polymers with High Conductivity and High Stretchability.ACS Appl. Mater. Interfaces2019, 11, 26185–26193. Google Scholar
- 47. Wang Y.; Zhu C.; Pfattner R.; Yan H.; Jin L.; Chen S.; Molina-Lopez F.; Lissel F.; Liu J.; Rabiah N. I.; Chen Z.; Chung J. W.; Linder C.; Toney M. F.; Murmann B.; Bao Z.A Highly Stretchable, Transparent, and Conductive Polymer.Sci. Adv.2017, 3, e1602076. Google Scholar
- 48. Bießmann L.; Saxena N.; Hohn N.; Hossain M. A.; Veinot J. G. C.; Müller-Buschbaum P.Highly Conducting, Transparent PEDOT:PSS Polymer Electrodes from Post-Treatment with Weak and Strong Acids.Adv. Electron. Mater.2019, 5, 1800654. Google Scholar
- 49. Kim J. Y.; Jung J. H.; Lee D. E.; Joo J.Enhancement of Electrical Conductivity of Poly(3,4-Ethylenedioxythiophene)/Poly(4-Styrenesulfonate) by a Change of Solvents.Synth. Met.2002, 126, 311–316. Google Scholar
- 50. Vosgueritchian M.; Lipomi D. J.; Bao Z.Highly Conductive and Transparent PEDOT:PSS Films with a Fluorosurfactant for Stretchable and Flexible Transparent Electrodes.Adv. Funct. Mater.2012, 22, 421–428. Google Scholar
- 51. Ouyang J.; Xu Q.; Chu C.-W.; Yang Y.; Li G.; Shinar J.On the Mechanism of Conductivity Enhancement in Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sulfonate) Film Through Solvent Treatment.Polymer2004, 45, 8443–8450. Google Scholar
- 52. Vaagensmith B.; Reza K. M.; Hasan M. N.; Elbohy H.; Adhikari N.; Dubey A.; Kantack N.; Gaml E.; Qiao Q.Environmentally Friendly Plasma-Treated PEDOT:PSS as Electrodes for ITO-Free Perovskite Solar Cells.ACS Appl. Mater. Interfaces2017, 9, 35861–35870. Google Scholar
- 53. Yildirim E.; Wu G.; Yong X.; Tan T. L.; Zhu Q.; Xu J.; Ouyang J.; Wang J.-S.; Yang S.-W.A Theoretical Mechanistic Study on Electrical Conductivity Enhancement of DMSO Treated PEDOT:PSS.J. Mater. Chem. C2018, 6, 5122–5131. Google Scholar
- 54. Wei Q.; Mukaida M.; Naitoh Y.; Ishida T.Morphological Change and Mobility Enhancement in PEDOT:PSS by Adding Co-solvents.Adv. Mater.2013, 25, 2831–2836. Google Scholar
- 55. Feig V. R.; Tran H.; Lee M.; Bao Z.Mechanically Tunable Conductive Interpenetrating Network Hydrogels That Mimic the Elastic Moduli of Biological Tissue.Nat. Commun.2018, 9, 2740. Google Scholar
- 56. Sun T.; Zhou B.; Zheng Q.; Wang L.; Jiang W.; Snyder G. J.Stretchable Fabric Generates Electric Power from Woven Thermoelectric Fibers.Nat. Commun.2020, 11, 572. Google Scholar
- 57. Gao Q.; Wang Y.; Wang P.; Shen M.; Li T.; Gao C.; Zhu J.Highly Stretchable, Conductive and Long-Term Stable PEDOT:PSS Fibers with Surface Arrays for Wearable Sensors.Adv. Eng. Mater.2022, 24, 2101448. Google Scholar
- 58. Sarabia-Riquelme R.; Shahi M.; Brill J. W.; Weisenberger M. C.Effect of Drawing on the Electrical, Thermoelectrical, and Mechanical Properties of Wet-Spun PEDOT:PSS Fibers.ACS Appl. Polym. Mater.2019, 1, 2157–2167. Google Scholar
- 59. Wen N.; Fan Z.; Yang S.; Zhao Y.; Cong T.; Xu S.; Zhang H.; Wang J.; Huang H.; Li C.; Pan L.Highly Conductive, Ultra-Flexible and Continuously Processable PEDOT:PSS Fibers with High Thermoelectric Properties for Wearable Energy Harvesting.Nano Energy2020, 78, 105361. Google Scholar
- 60. Chen H.; Xu H.; Luo M.; Wang W.; Qing X.; Lu Y.; Liu Q.; Yang L.; Zhong W.; Li M.; Wang D.Highly Conductive, Ultrastrong, and Flexible Wet-Spun PEDOT:PSS/Ionic Liquid Fibers for Wearable Electronics.ACS Appl. Mater. Interfaces2023, 15, 20346–20357. Google Scholar
- 61. Jo Y. J.; Kim S. Y.; Hyun J. H.; Park B.; Choy S.; Koirala G. R.; Kim T.Fibrillary Gelation and Dedoping of PEDOT:PSS Fibers for Interdigitated Organic Electrochemical Transistors and Circuits.Npj Flex. Electron.2022, 6, 31. Google Scholar
- 62. Dai T.; Qing X.; Lu Y.; Xia Y.Conducting Hydrogels with Enhanced Mechanical Strength.Polymer2009, 50, 5236–5241. Google Scholar
- 63. Wu Q.; Wei J.; Xu B.; Liu X.; Wang H.; Wang W.; Wang Q.; Liu W.A Robust, Highly Stretchable Supramolecular Polymer Conductive Hydrogel with Self-Healability and Thermo-Processability.Sci. Rep.2017, 7, 41566. Google Scholar
- 64. Lu B.; Yuk H.; Lin S.; Jian N.; Qu K.; Xu J.; Zhao X.Pure PEDOT:PSS Hydrogels.Nat. Commun.2019, 10, 1043. Google Scholar
- 65. Wang J.; Li Q.; Li K.; Sun X.; Wang Y.; Zhuang T.; Yan J.; Wang H.Ultra-High Electrical Conductivity in Filler-Free Polymeric Hydrogels Toward Thermoelectrics and Electromagnetic Interference Shielding.Adv. Mater.2022, 34, 2109904. Google Scholar
- 66. Wang H.; Zhuang T.; Wang J.; Sun X.; Wang Y.; Li K.; Dai X.; Guo Q.; Li X.; Chong D.; Chen B.; Yan J.Multifunctional Filler-Free PEDOT:PSS Hydrogels with Ultrahigh Electrical Conductivity Induced by Lewis-Acid-Promoted Ion Exchange.Adv. Mater.2023, 35, 2302919. Google Scholar
- 67. Chong J.; Sung C.; Nam K. S.; Kang T.; Kim H.; Lee H.; Park H.; Park S.; Kang J.Highly Conductive Tissue-like Hydrogel Interface Through Template-Directed Assembly.Nat. Commun.2023, 14, 2206. Google Scholar
- 68. Zhang D.; Tang Y.; Zhang Y.; Yang F.; Liu Y.; Wang X.; Yang J.; Gong X.; Zheng J.Highly Stretchable, Self-Adhesive, Biocompatible, Conductive Hydrogels as Fully Polymeric Strain Sensors.J. Mater. Chem. A2020, 8, 20474–20485. Google Scholar
- 69. Zhou T.; Yuk H.; Hu F.; Wu J.; Tian F.; Roh H.; Shen Z.; Gu G.; Xu J.; Lu B.; Zhao X.3D Printable High-Performance Conducting Polymer Hydrogel for All-Hydrogel Bioelectronic Interfaces.Nat. Mater.2023, 22, 895–902. Google Scholar
- 70. Jiang Y.; Zhang Z.; Wang Y.-X.; Li D.; Coen C.-T.; Hwaun E.; Chen G.; Wu H.-C.; Zhong D.; Niu S.; Wang W.; Saberi A.; Lai J.-C.; Wu Y.; Wang Y.; Trotsyuk A. A.; Loh K. Y.; Shih C.-C.; Xu W.; Liang K.; Zhang K.; Bai Y.; Gurusankar G.; Hu W.; Jia W.; Cheng Z.; Dauskardt R. H.; Gurtner G. C.; Tok J. B.-H.; Deisseroth K.; Soltesz I.; Bao Z.Topological Supramolecular Network Enabled High-Conductivity, Stretchable Organic Bioelectronics.Science2022, 375, 1411–1417. Google Scholar
- 71. Yuk H.; Lu B.; Lin S.; Qu K.; Xu J.; Luo J.; Zhao X.3D Printing of Conducting Polymers.Nat. Commun.2020, 11, 1604. Google Scholar
- 72. Xie X.; Xu Z.; Yu X.; Jiang H.; Li H.; Feng W.Liquid-in-Liquid Printing of 3D and Mechanically Tunable Conductive Hydrogels.Nat. Commun.2023, 14, 4289. Google Scholar
- 73. White H. S.; Kittlesen G. P.; Wrighton M. S.Chemical Derivatization of an Array of Three Gold Microelectrodes with Polypyrrole: Fabrication of a Molecule-Based Transistor.J. Am. Chem. Soc.1984, 106, 5375–5377. Google Scholar
- 74. Kim S.-M.; Kim C.-H.; Kim Y.; Kim N.; Lee W.-J.; Lee E.-H.; Kim D.; Park S.; Lee K.; Rivnay J.; Yoon M.-H.Influence of PEDOT:PSS Crystallinity and Composition on Electrochemical Transistor Performance and Long-Term Stability.Nat. Commun.2018, 9, 3858. Google Scholar
- 75. Khodagholy D.; Doublet T.; Quilichini P.; Gurfinkel M.; Leleux P.; Ghestem A.; Ismailova E.; Hervé T.; Sanaur S.; Bernard C.; Malliaras G. G.In Vivo Recordings of Brain Activity Using Organic Transistors.Nat. Commun.2013, 4, 1575. Google Scholar
- 76. Li W.; Jin J.; Xiong T.; Yu P.; Mao L.Fast-Scanning Potential-Gated Organic Electrochemical Transistors for Highly Sensitive Sensing of Dopamine in Living Rat Brain.Angew. Chem. Int. Ed.2022, 61, e202204134. Google Scholar
- 77. Corrado F.; Bruno U.; Prato M.; Carella A.; Criscuolo V.; Massaro A.; Pavone M.; Muñoz-García A. B.; Forti S.; Coletti C.; Bettucci O.; Santoro F.Azobenzene-Based Optoelectronic Transistors for Neurohybrid Building Blocks.Nat. Commun.2023, 14, 6760. Google Scholar
- 78. Rivnay J.; Inal S.; Salleo A.; Owens R. M.; Berggren M.; Malliaras G. G.Organic Electrochemical Transistors.Nat. Rev. Mater.2018, 3, 17086. Google Scholar
- 79. Deng Y.; Qi H.; Ma Y.; Liu S.; Zhao M.; Guo Z.; Jie Y.; Zheng R.; Jing J.; Chen K.; Ding H.; Lv G.; Zhang K.; Li R.; Cheng H.; Zhao L.; Sheng X.; Zhang M.; Yin L.A Flexible and Highly Sensitive Organic Electrochemical Transistor-Based Biosensor for Continuous and Wireless Nitric Oxide Detection.Proc. Natl. Acad. Sci.2022, 119, e2208060119. Google Scholar
- 80. Liu H.; Yang A.; Song J.; Wang N.; Lam P.; Li Y.; Law H. K.; Yan F.Ultrafast, Sensitive, and Portable Detection of COVID-19 IgG Using Flexible Organic Electrochemical Transistors.Sci. Adv.2021, 7, eabg8387. Google Scholar
- 81. Ji X.; Lin X.; Rivnay J.Organic Electrochemical Transistors as On-Site Signal Amplifiers for Electrochemical Aptamer-Based Sensing.Nat. Commun.2023, 14, 1665. Google Scholar
- 82. Yao C.; Xie C.; Lin P.; Yan F.; Huang P.; Hsing I.Organic Electrochemical Transistor Array for Recording Transepithelial Ion Transport of Human Airway Epithelial Cells.Adv. Mater.2013, 25, 6575–6580. Google Scholar
- 83. Van De Burgt Y.; Lubberman E.; Fuller E. J.; Keene S. T.; Faria G. C.; Agarwal S.; Marinella M. J.; Alec Talin A.; Salleo A.A Non-Volatile Organic Electrochemical Device as a Low-Voltage Artificial Synapse for Neuromorphic Computing.Nat. Mater.2017, 16, 414–418. Google Scholar
- 84. Keene S. T.; Lubrano C.; Kazemzadeh S.; Melianas A.; Tuchman Y.; Polino G.; Scognamiglio P.; Cinà L.; Salleo A.; Van De Burgt Y.; Santoro F.A Biohybrid Synapse with Neurotransmitter-Mediated Plasticity.Nat. Mater.2020, 19, 969–973. Google Scholar
- 85. Dai Y.; Dai S.; Li N.; Li Y.; Moser M.; Strzalka J.; Prominski A.; Liu Y.; Zhang Q.; Li S.; Hu H.; Liu W.; Chatterji S.; Cheng P.; Tian B.; McCulloch I.; Xu J.; Wang S.Stretchable Redox-Active Semiconducting Polymers for High-Performance Organic Electrochemical Transistors.Adv. Mater.2022, 34, 2201178. Google Scholar
- 86. Savagatrup S.; Chan E.; Renteria-Garcia S. M.; Printz A. D.; Zaretski A. V.; O’Connor T. F.; Rodriquez D.; Valle E.; Lipomi D. J.Plasticization of PEDOT:PSS by Common Additives for Mechanically Robust Organic Solar Cells and Wearable Sensors.Adv. Funct. Mater.2015, 25, 427–436. Google Scholar
- 87. Choong C.; Shim M.; Lee B.; Jeon S.; Ko D.; Kang T.; Bae J.; Lee S. H.; Byun K.; Im J.; Jeong Y. J.; Park C. E.; Park J.; Chung U.Highly Stretchable Resistive Pressure Sensors Using a Conductive Elastomeric Composite on a Micropyramid Array.Adv. Mater.2014, 26, 3451–3458. Google Scholar
- 88. Zhang F.; Zang Y.; Huang D.; Di C.; Zhu D.Flexible and Self-Powered Temperature–Pressure Dual-Parameter Sensors Using Microstructure-Frame-Supported Organic Thermoelectric Materials.Nat. Commun.2015, 6, 8356. Google Scholar
- 89. Liu H.; Zhang S.; Li Z.; Lu T. J.; Lin H.; Zhu Y.; Ahadian S.; Emaminejad S.; Dokmeci M. R.; Xu F.; Khademhosseini A.Harnessing the Wide-Range Strain Sensitivity of Bilayered PEDOT:PSS Films for Wearable Health Monitoring.Matter2021, 4, 2886–2901. Google Scholar
- 90. Wang Y.-F.; Sekine T.; Takeda Y.; Yokosawa K.; Matsui H.; Kumaki D.; Shiba T.; Nishikawa T.; Tokito S.Fully Printed PEDOT:PSS-Based Temperature Sensor with High Humidity Stability for Wireless Healthcare Monitoring.Sci. Rep.2020, 10, 2467. Google Scholar
- 91. Yu Y.; Peng S.; Blanloeuil P.; Wu S.; Wang C. H.Wearable Temperature Sensors with Enhanced Sensitivity by Engineering Microcrack Morphology in PEDOT:PSS–PDMS Sensors.ACS Appl. Mater. Interfaces2020, 12, 36578–36588. Google Scholar
- 92. Nemani S. K.; Chen D.; Mohamed M. H.; Sojoudi H.Stretchable and Hydrophobic Electrochromic Devices Using Wrinkled Graphene and PEDOT:PSS.J. Nanomater.2018, 2018, 1–10. Google Scholar
- 93. Yin L.; Cao M.; Kim K. N.; Lin M.; Moon J.-M.; Sempionatto J. R.; Yu J.; Liu R.; Wicker C.; Trifonov A.; Zhang F.; Hu H.; Moreto J. R.; Go J.; Xu S.; Wang J.A Stretchable Epidermal Sweat Sensing Platform with an Integrated Printed Battery and Electrochromic Display.Nat. Electron.2022, 5, 694–705. Google Scholar
- 94. Matsuhisa N.; Niu S.; O’Neill S. J. K.; Kang J.; Ochiai Y.; Katsumata T.; Wu H.-C.; Ashizawa M.; Wang G.-J. N.; Zhong D.; Wang X.; Gong X.; Ning R.; Gong H.; You I.; Zheng Y.; Zhang Z.; Tok J. B.-H.; Chen X.; Bao Z.High-Frequency and Intrinsically Stretchable Polymer Diodes.Nature2021, 600, 246–252. Google Scholar
- 95. Obien M. E. J.; Deligkaris K.; Bullmann T.; Bakkum D. J.; Frey U.Revealing Neuronal Function Through Microelectrode Array Recordings.Front. Neurosci.2015, 8, 423. Google Scholar
- 96. Liang Y.; Offenhäusser A.; Ingebrandt S.; Mayer D.PEDOT:PSS-Based Bioelectronic Devices for Recording and Modulation of Electrophysiological and Biochemical Cell Signals.Adv. Healthc. Mater.2021, 10, 2100061. Google Scholar
- 97. Zhou W.; Jiang Y.; Xu Q.; Chen L.; Qiao H.; Wang Y.-X.; Lai J.-C.; Zhong D.; Zhang Y.; Li W.; Du Y.; Wang X.; Lei J.; Dong G.; Guan X.; Ma S.; Kang P.; Yuan L.; Zhang M.; Tok J. B.-H.; Li D.; Bao Z.; Jia W.Soft and Stretchable Organic Bioelectronics for Continuous Intraoperative Neurophysiological Monitoring during Microsurgery.Nat. Biomed. Eng.2023, 7, 1270–1281. Google Scholar
- 98. Liu J.; Zhang X.; Liu Y.; Rodrigo M.; Loftus P. D.; Aparicio-Valenzuela J.; Zheng J.; Pong T.; Cyr K. J.; Babakhanian M.; Hasi J.; Li J.; Jiang Y.; Kenney C. J.; Wang P. J.; Lee A. M.; Bao Z.Intrinsically Stretchable Electrode Array Enabled in Vivo Electrophysiological Mapping of Atrial Fibrillation at Cellular Resolution.Proc. Natl. Acad. Sci.2020, 117, 14769–14778. Google Scholar
- 99. Khodagholy D.; Gelinas J. N.; Zhao Z.; Yeh M.; Long M.; Greenlee J. D.; Doyle W.; Devinsky O.; Buzsáki G.Organic Electronics for High-Resolution Electrocorticography of the Human Brain.Sci. Adv.2016, 2, e1601027. Google Scholar
- 100. Sessolo M.; Khodagholy D.; Rivnay J.; Maddalena F.; Gleyzes M.; Steidl E.; Buisson B.; Malliaras G. G.Easy-to-Fabricate Conducting Polymer Microelectrode Arrays.Adv. Mater.2013, 25, 2135–2139. Google Scholar
- 101. Han L.; Lu X.; Liu K.; Wang K.; Fang L.; Weng L.-T.; Zhang H.; Tang Y.; Ren F.; Zhao C.; Sun G.; Liang R.; Li Z.Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization.ACS Nano2017, 11, 2561–2574. Google Scholar
- 102. Khodagholy D.; Gelinas J. N.; Thesen T.; Doyle W.; Devinsky O.; Malliaras G. G.; Buzsáki G.NeuroGrid: Recording Action Potentials from the Surface of the Brain.Nat. Neurosci.2015, 18, 310–315. Google Scholar
- 103. Spyropoulos G. D.; Gelinas J. N.; Khodagholy D.Internal Ion-Gated Organic Electrochemical Transistor: A Building Block for Integrated Bioelectronics.Sci. Adv.2019, 5, eaau7378. Google Scholar
- 104. Yuk H.; Lu B.; Zhao X.Hydrogel Bioelectronics.Chem. Soc. Rev.2019, 48, 1642–1667. Google Scholar
- 105. Spira M. E.; Hai A.Multi-Electrode Array Technologies for Neuroscience and Cardiology.Nat. Nanotechnol.2013, 8, 83–94. Google Scholar
- 106. Wang W.; Jiang Y.; Zhong D.; Zhang Z.; Choudhury S.; Lai J.-C.; Gong H.; Niu S.; Yan X.; Zheng Y.; Shih C.-C.; Ning R.; Lin Q.; Li D.; Kim Y.-H.; Kim J.; Wang Y.-X.; Zhao C.; Xu C.; Ji X.; Nishio Y.; Lyu H.; Tok J. B.-H.; Bao Z.Neuromorphic Sensorimotor Loop Embodied by Monolithically Integrated, Low-Voltage, Soft e-Skin.Science2023, 380, 735–742. Google Scholar
- 107. Liu Y.; Liu J.; Chen S.; Lei T.; Kim Y.; Niu S.; Wang H.; Wang X.; Foudeh A. M.; Tok J. B.-H.; Bao Z.Soft and Elastic Hydrogel-Based Microelectronics for Localized Low-Voltage Neuromodulation.Nat. Biomed. Eng.2019, 3, 58–68. Google Scholar